Method for producing cefdinir active ester
A technology of cefdinir active ester and inert solvent, applied in the field of production of cefdinir active ester, can solve problems such as unqualified quality and low purity, and achieve the effects of reducing dosage, high product purity and few process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] At room temperature, add 20.16g of potassium tert-butoxide, 12.6g of tetramethylpropylenediamine and 400ml of acetonitrile into the three-necked flask, stir for 30min, throw in 50g of demethylaminothioxamic acid ethyl ester, stir for 4h, cool down to 25°C, and dropwise add 29.4g of acetic anhydride , with a pH value of about 6.1, reacted at 40°C for 1 hour to obtain an organic amine salt solution of acetylated demethylthioxamic acid;
[0042] Cool down to 15°C, add 23.0g triethyl phosphite dropwise, add 56.5g DM within 30min, keep warm for 1h, cool down to below 0°C, and filter with suction to obtain the crude cefdinir active ester;
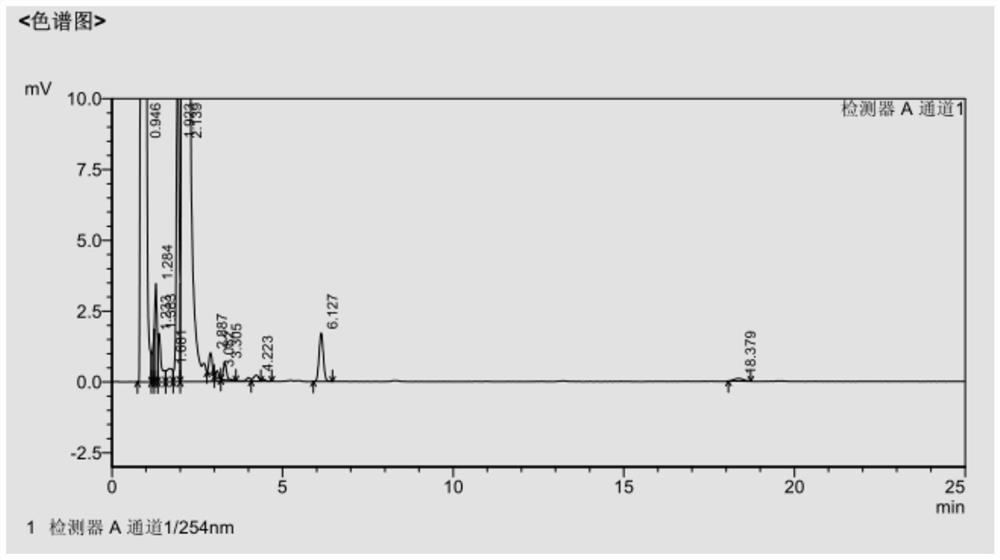
[0043] The filter cake was dissolved in 200ml DMF, 360ml methanol was added dropwise for 1 hour, suction filtered, and vacuum-dried at 40°C for 4 hours to obtain 65.5g of cefdinir active ester with a molar yield of 74.02% and a purity of 99.45%. The HPLC data are shown in Table 1, and the spectrograms are shown in figure 1 .
[0044] Tab...
Embodiment 2
[0048] At room temperature, add 24.80g of potassium tert-butoxide, 18g of triethylamine and 400ml of acetonitrile into the three-necked flask, stir for 30min, throw in 50g of ethyl demethylaminothiaxate, stir for 4h, then cool down to 25°C and add 29.8g of acetic anhydride dropwise, the pH value is about 8.4, react at 40°C for 1 hour to obtain an organic amine salt solution of acetylated demethiazoxamic acid;
[0049] Cool down to 15°C, add 28.2g triethyl phosphite dropwise, add 56.8g DM within 60min, keep warm for 1h, cool down to below 0°C, and filter with suction to obtain the crude product of cefdinir active ester;
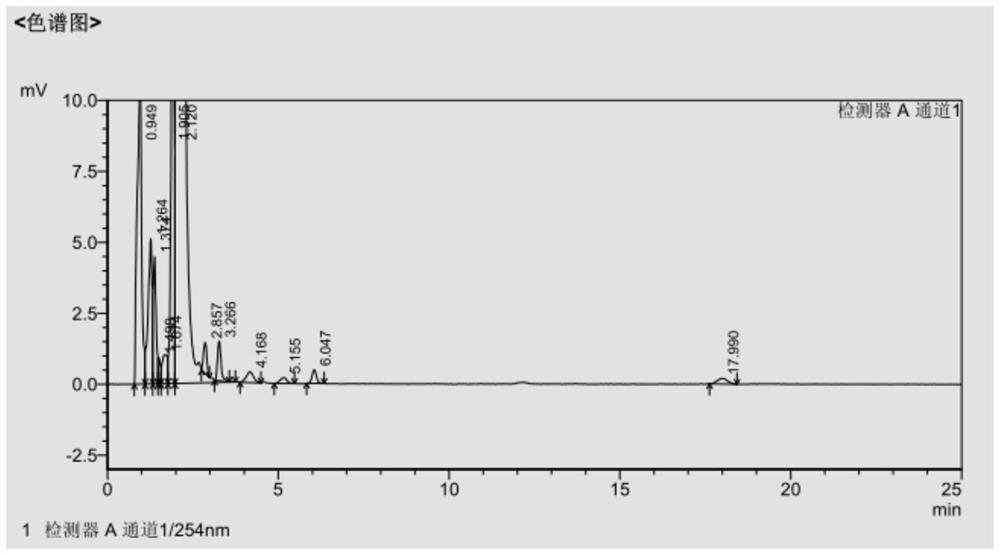
[0050] The filter cake was dissolved with 300ml DMF, 500ml methanol was added dropwise for 1 hour, suction filtered, and vacuum-dried at 40°C for 4 hours to obtain 60.1g of cefdinir active ester with a molar yield of 68.33% and a purity of 99.58%. The HPLC data are shown in Table 2, and the spectrograms are shown in figure 2 .
[0051] Table 2 Example 2 HPL...
Embodiment 3
[0054] At room temperature, add 13.9g of sodium methoxide, 16g of propylamine and 400ml of acetonitrile into the three-necked flask, stir for 30min, throw in 50g of ethyl demethylthioxamate, stir for 4h, then cool down to 25°C and add 30g of acetic anhydride dropwise, the pH value is about 5.2, at 40°C Reaction for 1h to obtain the organic amine salt solution of acetylated demethiazoxamic acid;
[0055]Cool down to 15°C, add 26.8g of triethyl phosphite dropwise, add 56.5g of DM within 90min, keep warm for 1h, cool down to below 0°C, and filter with suction to obtain the crude product of cefdinir active ester;
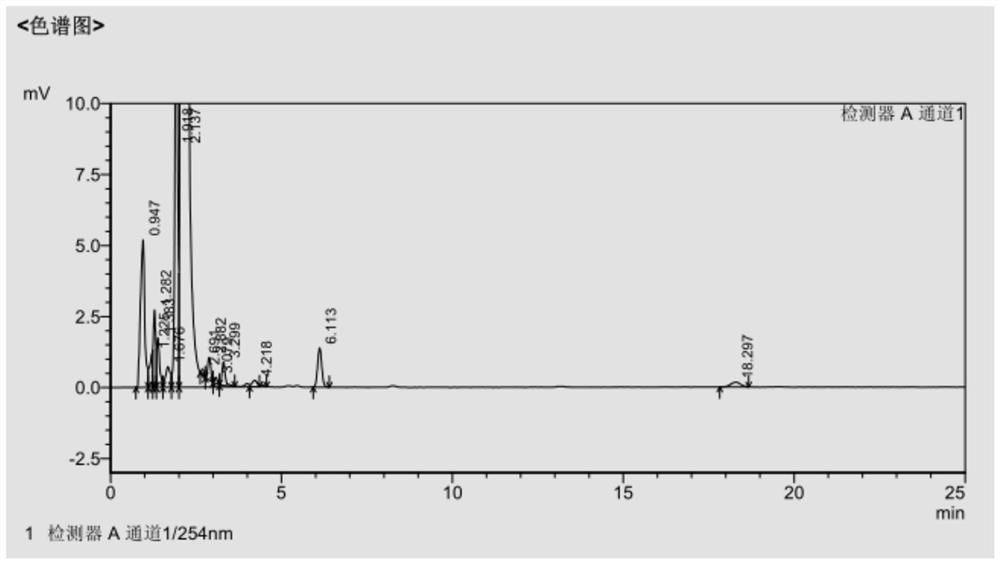
[0056] Dissolve the filter cake with 400ml of DMF, add 600ml of methanol dropwise for 1h, filter with suction, and dry in vacuum at 40°C for 4h to obtain 57.5g of cefdinir active ester with a molar yield of 65.48% and a purity of 99.59%. The HPLC data are shown in Table 3, and the spectrograms are shown in image 3 .
[0057] Table 3 Example 3 HPLC data sheet
[0058...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com