Derivatization reagent and application thereof
A technology of derivatization reagents and chemical bonds, which is applied in the field of analysis and detection, can solve the problems of few types of derivatization reagents, difficult quantitative analysis, and uneven derivatization efficiency, so as to avoid co-competitive ionization, improve the ionization efficiency of mass spectrometry, and widely The effect of developing application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
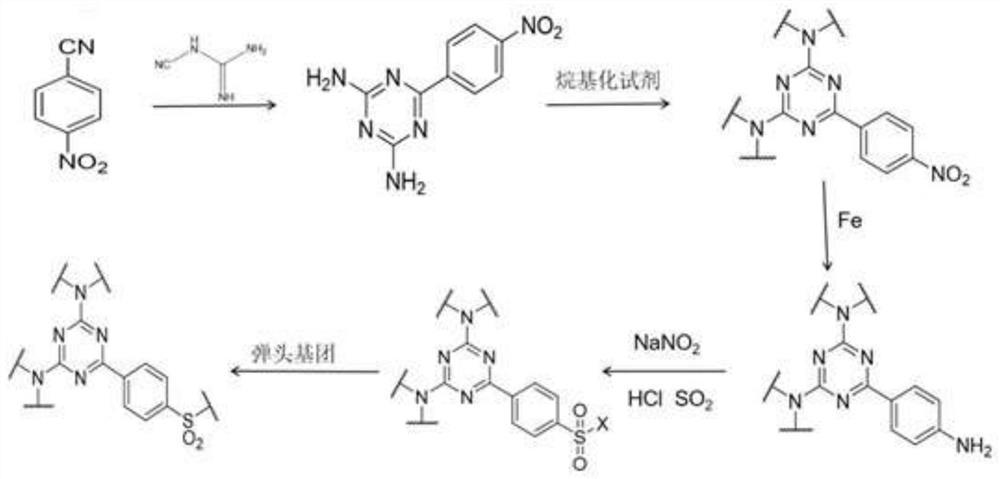
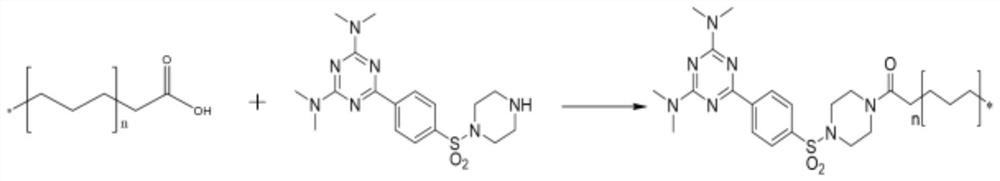
[0085] Example 1: Synthesis of compound 1 in formula II: N2, N2, N4, N4-tetramethyl-2,4-diamino-6-(4-phenylsulfonylpiperazine)-1,3,5- Triazine (TMTPP):
[0086] (1), the preparation of intermediate 2,4-diamino-6-(4-nitrophenyl)-1,3,5-triazine:
[0087] Add 2g of p-nitrobenzonitrile, 1.3g of dicyandiamide and 0.3g of potassium hydroxide into the three-necked flask, add 300mL of absolute ethanol, put in a magnet, place it in an oil bath at 78°C, and reflux for 3 hours. After the reaction is completed, cool to room temperature, add absolute ethanol to wash, filter, precipitate solid, evaporate and dry in the air, the obtained solid is the intermediate 2,4-diamino-6-(4-nitrophenyl)-1,3 , 5-triazine, the productive rate is 96.7%;
[0088] (2), preparation of intermediate N2, N2, N4, N4-tetramethyl-2,4-diamino-6-(4-nitrophenyl)-1,3,5-triazine:
[0089] Add 1.48g sodium hydride and 80mL N,N-dimethylformamide into the three-necked flask, then add 2g 2,4-diamino-6-(4-nitrophenyl)-1,...
Embodiment 2
[0096] Example 2, Compound 1 is used for the analysis and detection of acylcarnitine:
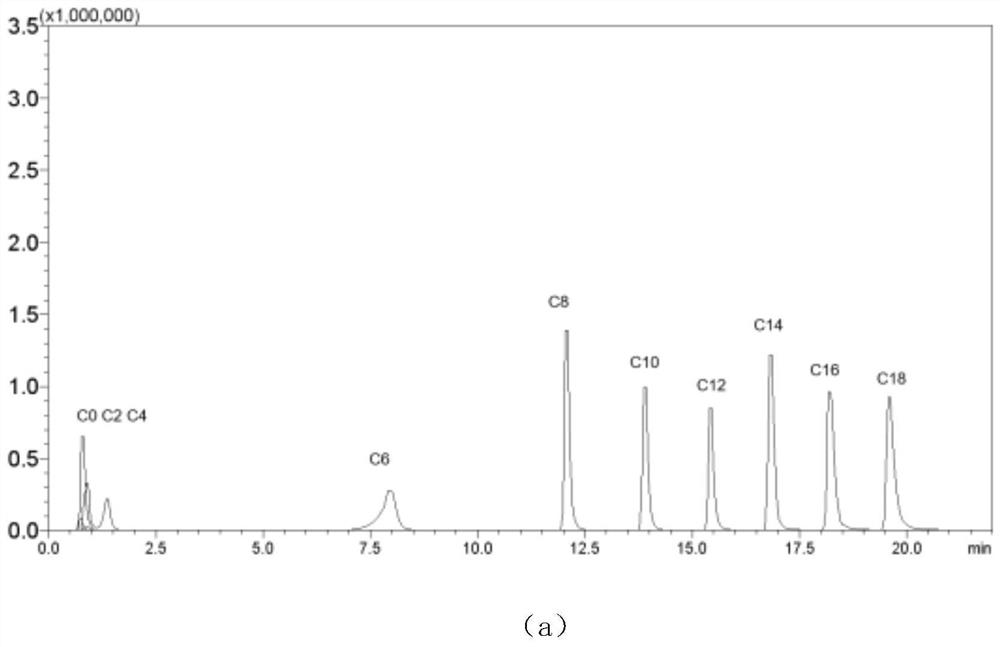
[0097] Take the blank control, 5-dimethylaminonaphthalene-1-sulfonylpiperazine and N2, N2, N4, N4-tetramethyl-2,4-diamino-6-(4-phenylsulfonylpiperazine) respectively -1,3,5-triazine was prepared as 9mM, and 20uL of it was taken respectively, mixed with 20uL of 500ng / mL 11 straight-chain acylcarnitine mixed standard solution, 20uL of 3mM HATU and 40uL of acetonitrile / water (1:1), Vortex for 5 minutes, mix well, heat the reaction at 47°C for 150 minutes, centrifuge at 20,000g for 10 minutes, take 20uL of the supernatant and inject it into HPLC-MS / MS for analysis;
[0098] The results show that it can be seen that the underivatized blank control, the short-chain acylcarnitine does not retain, the same concentration of N2, N2, N4, N4-tetramethyl-2,4-diamino-6-(4-benzene The mass spectrum response value of sulfonylpiperazine)-1,3,5-triazine is 3 to 4 times that of 5-dimethylaminonaphthalene-1-s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com