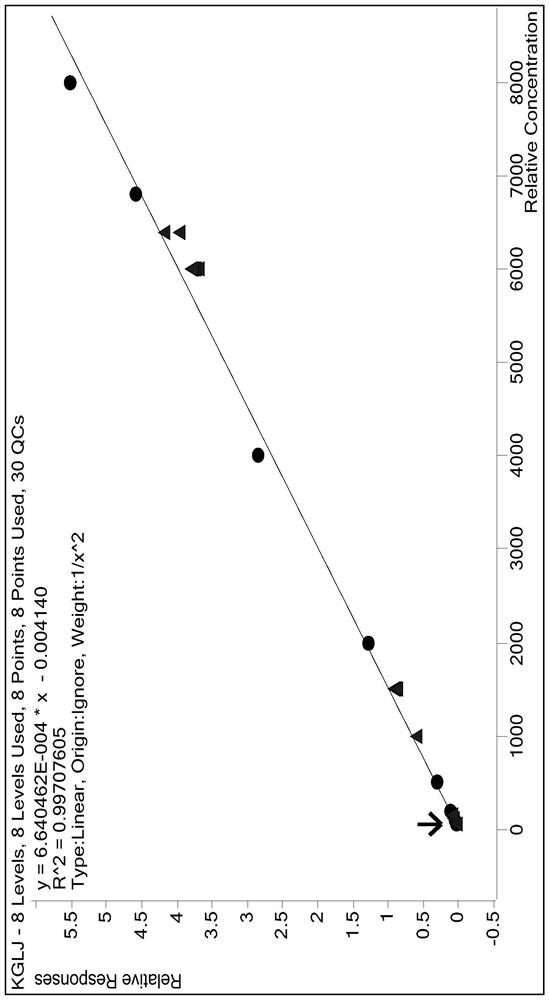
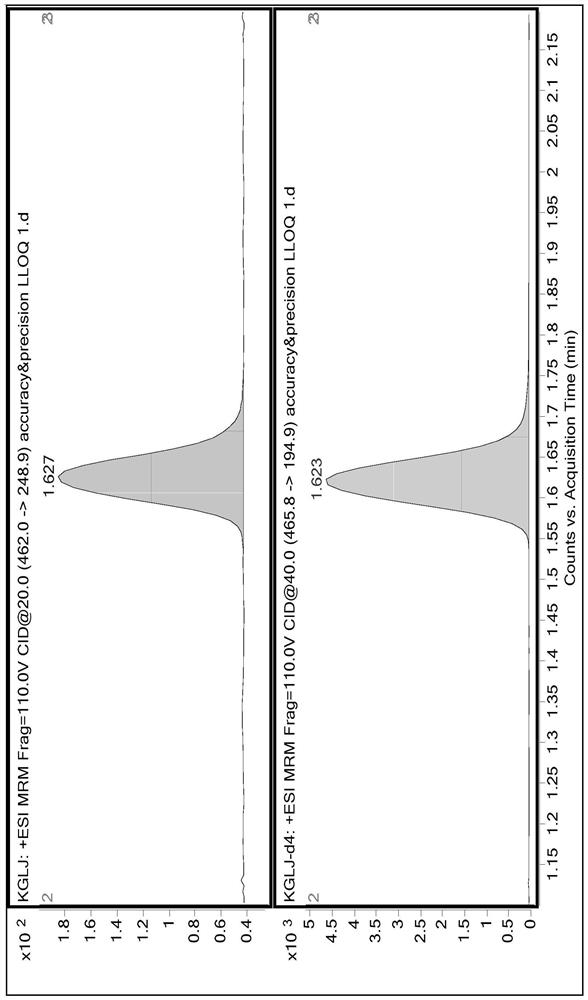
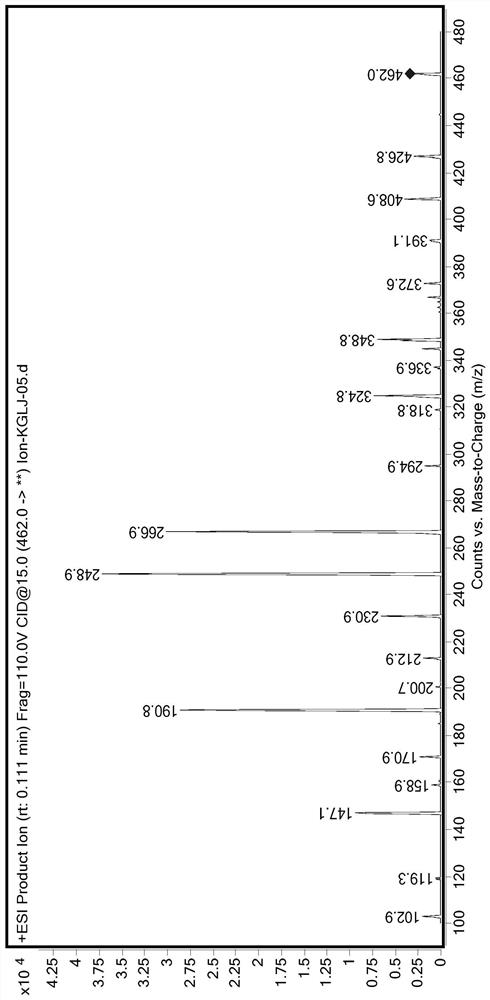
Method for measuring concentration of canagliflozin in human plasma by LC-MS/MS
A technology of human plasma concentration, applied in the field of medical testing, can solve the problems of complicated operation steps and large amount of plasma, and achieve the effects of saving project cost, small consumption of plasma, and accurate and reliable results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] 1. Solution preparation
[0104] Mobile phase A (10mM ammonium acetate aqueous solution)
[0105] Weigh about 0.7708g of ammonium acetate and dissolve it in 1L of ultrapure water, mix well and set aside.
[0106] Mobile phase B (acetonitrile)
[0107] Measure 2L of acetonitrile (Merck) in a 2L solvent bottle for use
[0108] Needle washing solution (methanol: water, 90:10, V / V)
[0109] Separately take 900mL of methanol and 100mL of ultrapure water and transfer them to an appropriate solvent bottle, mix well, and set aside.
[0110] Preparation of working fluid diluent (50% acetonitrile aqueous solution)
[0111] Separate 250ml of ultrapure water and 250ml of acetonitrile into an appropriate solvent bottle, and mix well.
[0112] Precipitating agent (acetonitrile)
[0113] Measure 500mL of acetonitrile into an appropriate solvent bottle for use.
[0114] 2. Standard solution preparation:
[0115] Preparation of standard reference substance stock solution
[011...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com