Polyvanadate alkoxy derivative with insulin sensitization activity as well as preparation method and application of polyvanadate alkoxy derivative
A technology of polyvanadate and insulin sensitization, which is applied in the field of animal medicine and can solve the problems of long-term toxicity and low oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
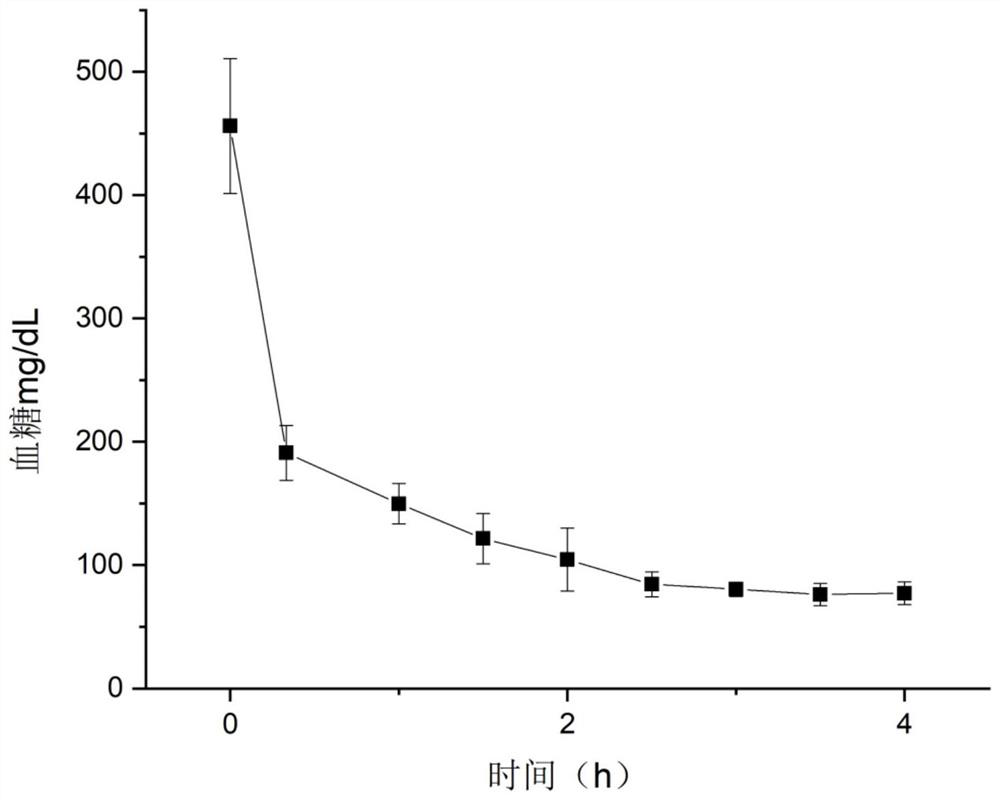
Examples
Embodiment 1
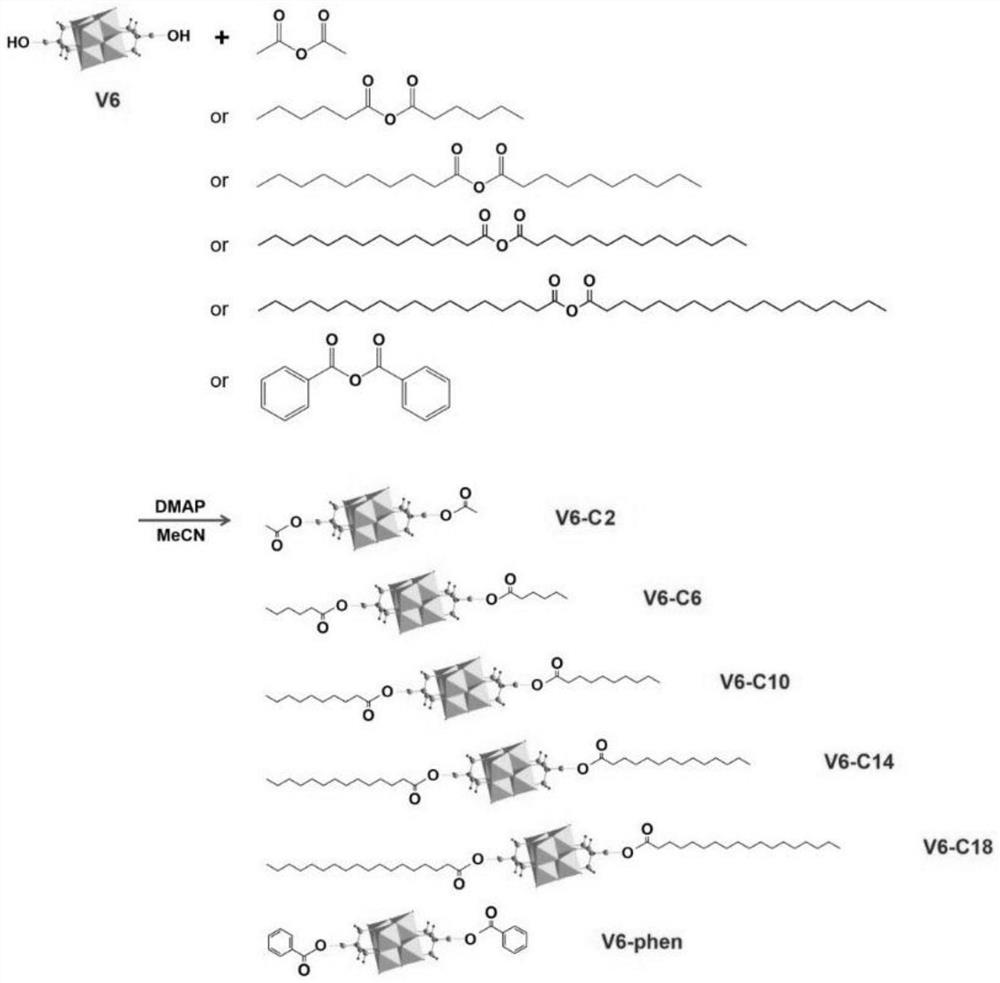
[0055] Synthesis of POV derivatives. 1mmol hexavanadate ((Bu 4 N) 2 [V 6 o 13 {(OCH 2 ) 3 CCH 2 OH} 2 ]) and 2mmol acetic anhydride were mixed evenly, and then 0.1g of DMAP, 2mmol triethylamine and 20mLCH were added 3 CN, the reaction was stirred at 80°C for 48h. After cooling to room temperature, the product mixture obtained by the reaction was poured into 50 mL of deionized water, and the red precipitate was collected by filtration. Exchanged by cation exchange resin to H + For cationic polyvanadate alkoxy derivatives, H[V 6 o 13 {(OCH 2 ) 3 CCH 2 OCOCH 3} 2 ].
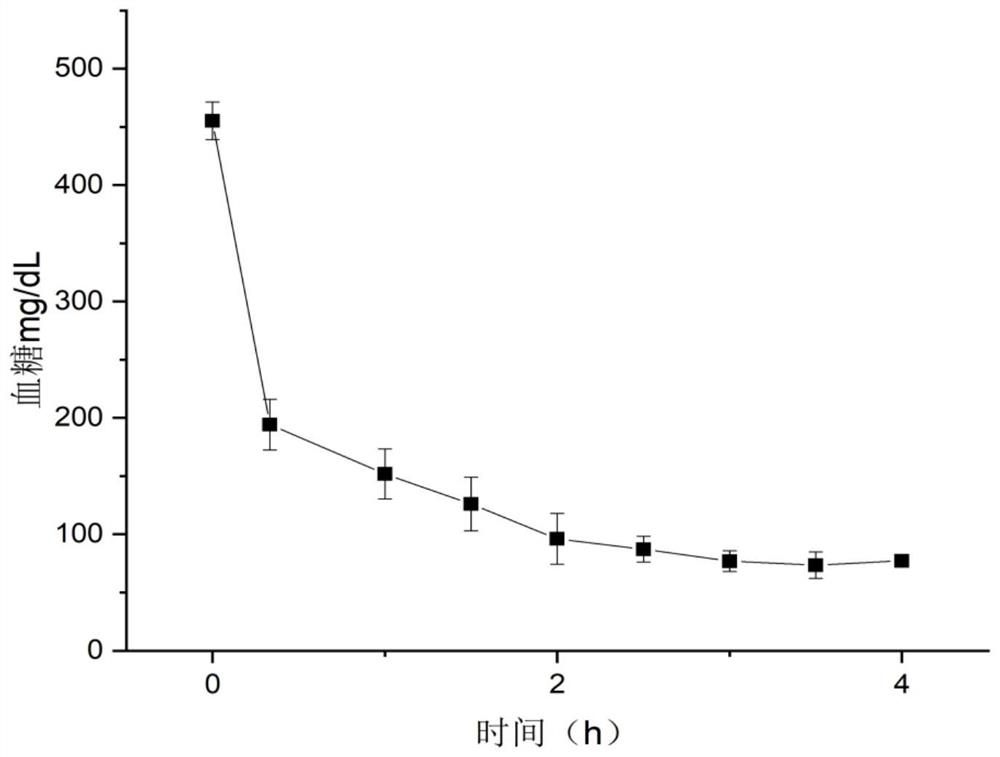
Embodiment 2
[0057] Synthesis of POV derivatives. 1mmol hexavanadate ((Bu 4 N) 2 [V 6 o 13 {(OCH 2 ) 3 CCH 2 OH} 2 ]), and 2mmol acetic anhydride are mixed evenly, then add 0.1g DMAP, 2mmol triethylamine and 20mLCH 3 CN, the reaction was stirred at 80°C for 48h. After cooling to room temperature, the product mixture obtained by the reaction was poured into 50 mL of deionized water, and the red precipitate was collected by filtration. Exchanged by cation exchange resin to H + The polyvanadate alkoxy derivatives that are cationic, and then exchanged with Al by ion exchange 3+ As a cationic polyvanadate alkoxy derivative, Al 2 [V 6 o 13 {(OCH 2 ) 3 CCH 2 OCOCH 3} 2 ] 3 , denoted as V6-C2.
Embodiment 3
[0059] Synthesis of POV derivatives. 1mmol hexavanadate ((Bu 4 N) 2 [V 6 o 13 {(OCH 2 ) 3 CCH 2 OH} 2 ]), and 2mmol decanoic anhydride were mixed evenly, then added 0.25g of DMAP, 2mmol triethylamine and 20mLCH 3 CN, the reaction was stirred at 80°C for 48h. After cooling to room temperature, the product mixture obtained by the reaction was poured into 50 mL of deionized water, and the red precipitate was collected by filtration. exchanged with Na by cation exchange resin + As a cationic polyvanadate alkoxy derivative, Na[V 6 o 13 {(OCH 2 ) 3 CCH 2 OCO(CH 2 ) 8 CH 3} 2 ], recorded as V6-C10.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com