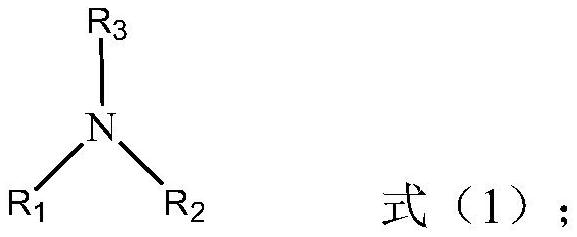
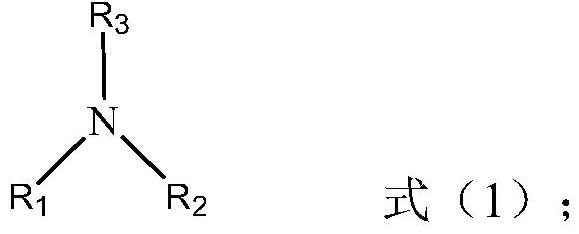Method for removing water from ionic liquid alcoholic solution
A technology of ionic liquids and acidic ionic liquids, applied in chemical instruments and methods, preparation of organic compounds, catalysts of organic compounds/hydrides/coordination complexes, etc. Poor performance and other problems, to achieve the effect of reducing energy consumption and meeting operating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Take by weighing 300g ethanol, 21g water and 9g 1-methyl-3-propanesulfonic acid imidazolium bisulfate ([MPSIm][HSO 4 ]) is configured as an initial ionic liquid alcohol solution, and the pH value of the ionic liquid alcohol solution is 0.71 at this moment. Add 1.31 g of diethylamine to the prepared ionic liquid alcohol solution at 10° C. to adjust the pH of the system to 5.21. The adjusted ionic liquid alcohol solution enters the pervaporation unit, wherein the pervaporation membrane is a molecular sieve membrane. Under the operating conditions of 40° C. and permeation side pressure of 200 Pa, the dehydration was circulated in the pervaporation unit for 4 hours to obtain the ionic liquid alcohol solution after water removal. The ionic liquid alcohol solution after water removal was heated to reflux at 60° C., the temperature of the condensation column was 40° C., and the final product was obtained after 1 hour. The water content in the system measured by a Karl Fische...
Embodiment 2
[0042] Take by weighing 300g ethanol, 21g water and 9g 1-methyl-3-propanesulfonic acid imidazolium bisulfate ([MPSIm][HSO 4 ]) is configured as an initial ionic liquid alcohol solution, and the pH value of the ionic liquid alcohol solution is 0.71 at this moment. Add 3.00 g of triethylamine to the prepared ionic liquid alcohol solution at 10° C. to adjust the pH of the system to 6.72. The adjusted ionic liquid alcohol solution enters the pervaporation unit, wherein the pervaporation membrane is a molecular sieve membrane. Under the operating conditions of 40° C. and permeation side pressure of 200 Pa, the dehydration was circulated in the pervaporation unit for 4 hours to obtain the ionic liquid alcohol solution after water removal. The ionic liquid alcohol solution after water removal was heated to reflux at 65° C., the temperature of the condensation column was 40° C., and the final product was obtained after 1 hour. The water content in the system measured by a Karl Fisch...
Embodiment 3
[0044] Take by weighing 300g ethanol, 21g water and 9g 1-methyl-3-propanesulfonic acid imidazolium bisulfate ([MPSIm][HSO 4 ]) is configured as an initial ionic liquid alcohol solution, and the pH value of the ionic liquid alcohol solution is 0.71 at this moment. Add 2.87 g of dimethyl sulfoxide to the prepared ionic liquid alcohol solution at 10° C. to adjust the pH of the system to 7.89. The adjusted ionic liquid alcohol solution enters the pervaporation unit, wherein the pervaporation membrane is a molecular sieve membrane. Under the operating conditions of 40° C. and permeation side pressure of 200 Pa, the dehydration was circulated in the pervaporation unit for 4 hours to obtain the ionic liquid alcohol solution after water removal. The ionic liquid alcohol solution after water removal was heated to reflux at 60° C., the temperature of the condensation column was 40° C., and the final product was obtained after 1 hour. The water content in the system measured by a Karl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com