Synthesis method of alpha or beta-substituted aromatic ketone
A synthesis method and technology for aromatic ketones are applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., and can solve the problems of low atom economy, inconvenient operation, and high price of dibutylboron trifluoromethanesulfonate reagent. and other problems, to achieve the effect of high atomic economy, convenient operation and large-scale industrialization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
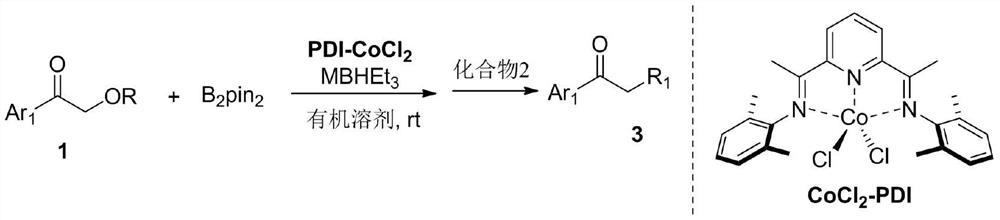
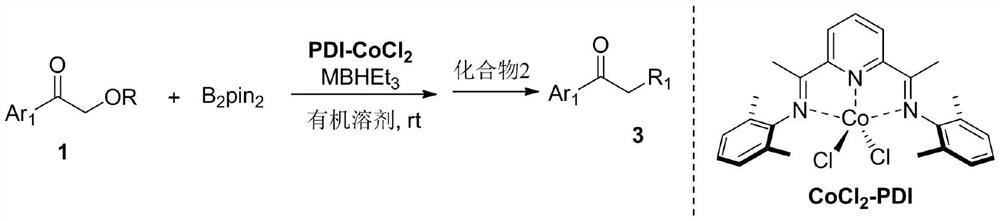
[0023] A kind of synthetic method of aromatic ketone substituted in α or β position, specifically as follows:
[0024]
[0025] At room temperature, under nitrogen, in a 10mL Schlenk reaction tube, add PDI-CoCl 2 (0.01mmol), Et 2 O (1 mL), Compound 1a (1 mmol), B 2 pin 2 (1mmol) and NaBHEt 3 (0.02mmol), the reaction solution was stirred at room temperature for 1 hour, compound 2a (1.5mmol) was added, continued to stir at room temperature for 1 hour, and column chromatography separated to obtain compound 3a as a colorless oily liquid with a yield of 92%.
[0026] The NMR characterization data of compound 3a are:
[0027] 1 H NMR (CDCl 3 ,400MHz):δ7.90-7.99(m,2H),7.53-7.63(m,1H),7.41–7.50(m,4H),7.34-7.42(m,2H),7.26-7.35(m,1H) , 5.38 (ddd, J = 6.2, 6.2, 2.4Hz, 1H), 3.65 (d, J = 2.4Hz, 1H), 3.34-3.42 (m, 2H).
Embodiment 2
[0029] Basically the same as embodiment 1, the difference is: adopt LiBHEt 3 instead of NaBHEt 3 , the yield of compound 3a was 85%.
Embodiment 3
[0031] It is basically the same as Example 1, the difference is: adopt THF instead of Et 2 O, the yield of compound 3a was 66%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com