Preparation method of isoniazid
A technology of isoniazid and isonicotinic acid, applied in organic chemistry and other directions, can solve problems such as difficult operability and controllability, narrow temperature range, etc., and achieves wide reaction temperature range, improved yield and quality, and large selection. range effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
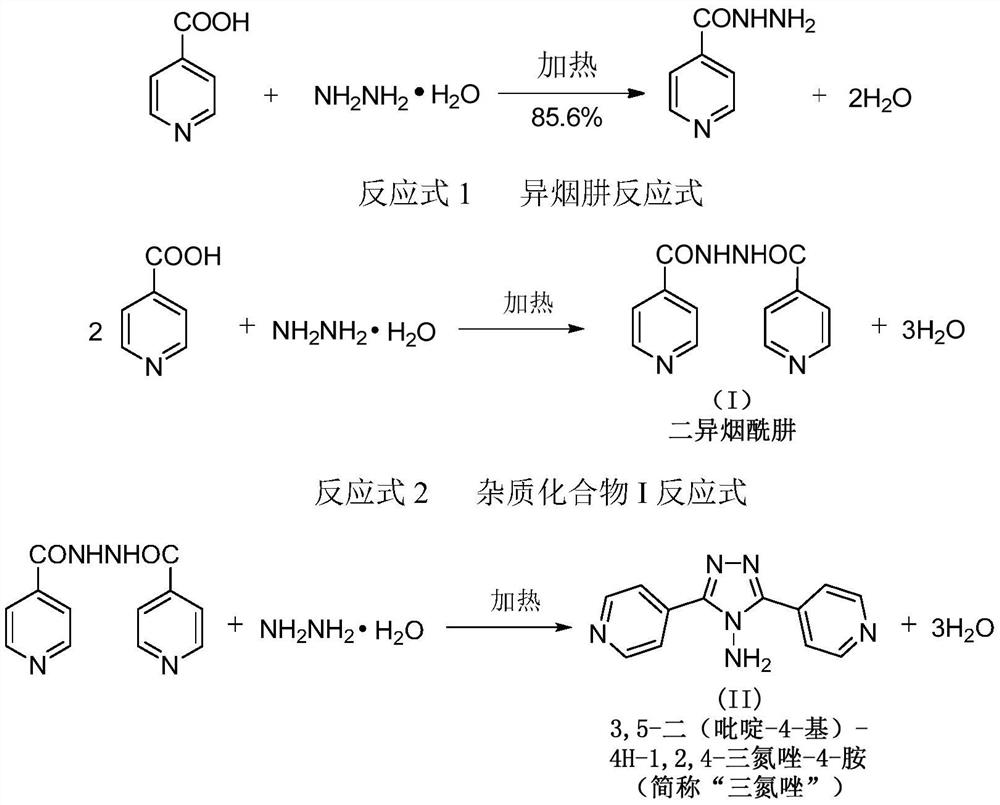
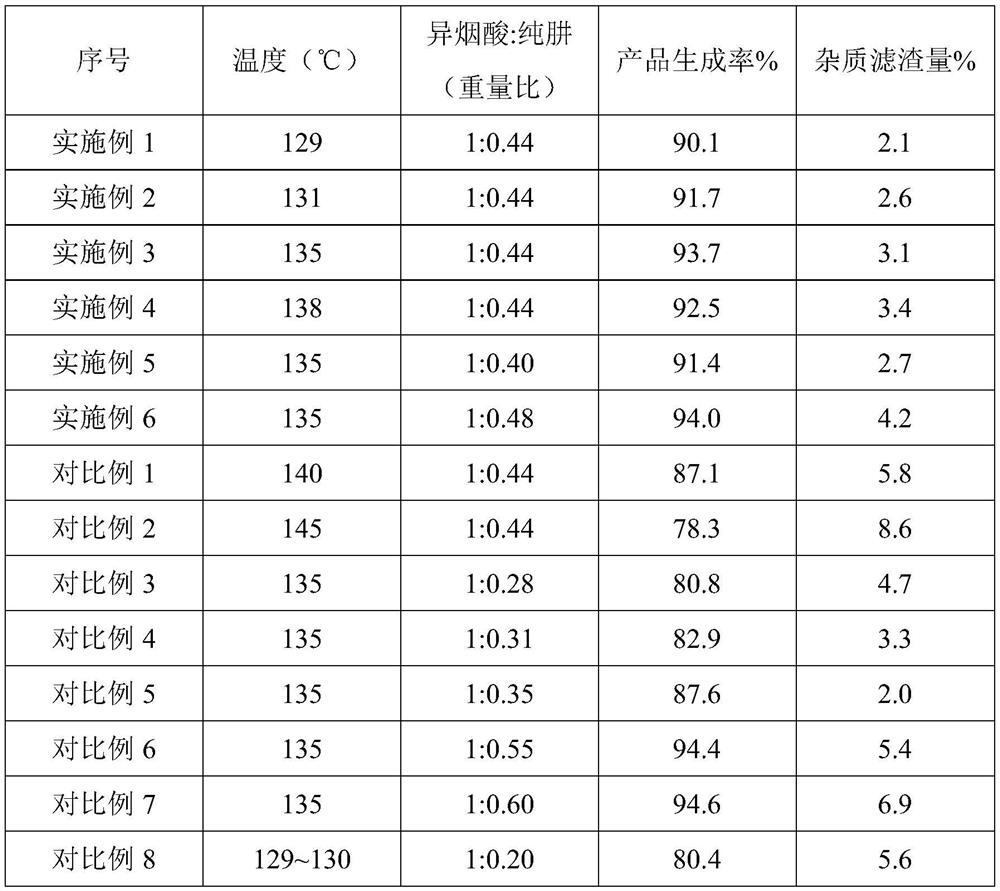
[0052] Embodiment 1 A kind of preparation of isoniazid
[0053] S1, the synthesis of isoniazid
[0054] Dissolve 150kg of isonicotinic acid in 258kg of 40% hydrazine hydrate, distill under reduced pressure to 79-82°C, then change to normal pressure environment, rapidly raise the temperature of the reaction system to 129°C, react for 3 hours, add 193.5kg of purified water to quench The reaction was carried out to obtain the crude product suspension of isoniazid.
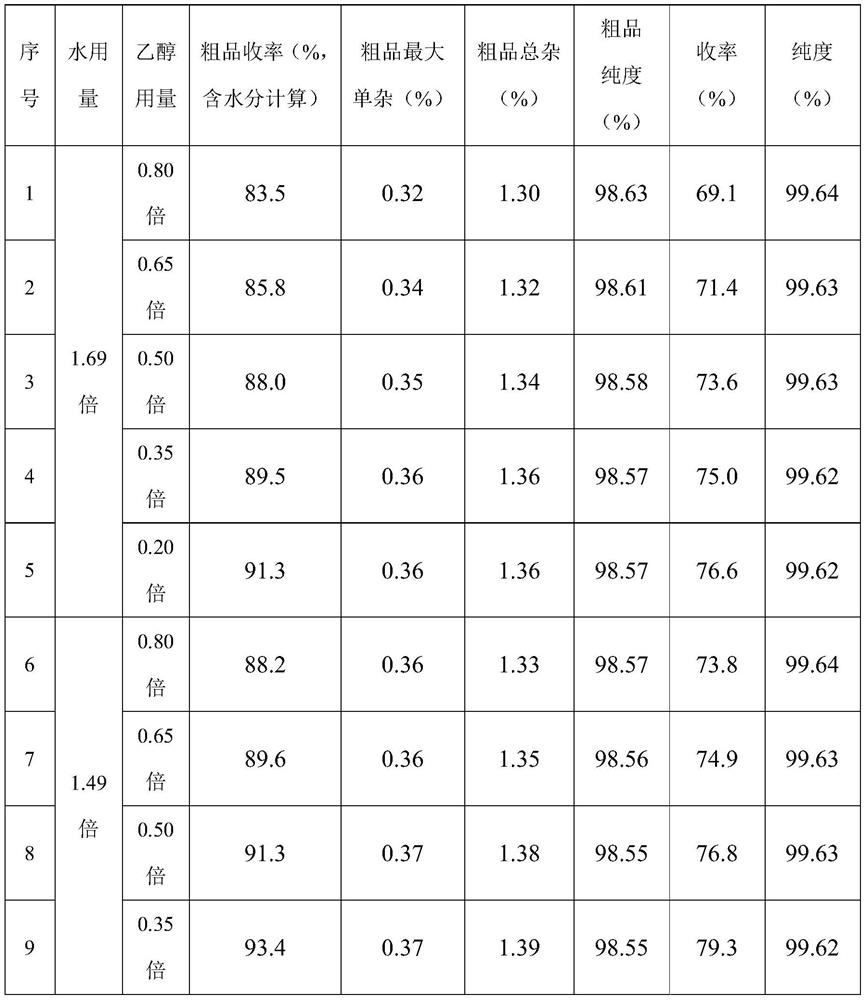
[0055] S2, refining of isoniazid
[0056] S2-1. Decolorization: Cool the crude isoniazid suspension obtained in the above step S1. at room temperature for 4 hours, heat the cooled crude suspension to 70-80°C to dissolve, add 2% activated carbon, and stir for 15 minutes. Filter while hot with a filter cloth with a mesh number of 325, quickly transfer the filtrate to the crystallization kettle, gradually cool down and crystallize until the system temperature is 5-10°C, control the cooling and crystallization time for ...
Embodiment 7
[0070] Embodiment 7 A kind of preparation of isoniazid
[0071] S1, the synthesis of isoniazid
[0072] Dissolve 150kg of isonicotinic acid in 258kg of 40% hydrazine hydrate, distill under reduced pressure to 79-82°C, then change to normal pressure environment, rapidly raise the temperature of the reaction system to 135°C, react for 3 hours, add 223.5kg of purified water to quench The reaction was carried out to obtain the crude product suspension of isoniazid.
[0073] S2, refining of isoniazid
[0074] S2-1. Decolorization: Cool the crude isoniazid suspension obtained in the above step S1. at room temperature for 4 hours, heat the cooled crude suspension to 70-80°C to dissolve, add 2% activated carbon, and stir for 15 minutes. Filter while hot with a filter cloth with a mesh number of 325, quickly transfer the filtrate to the crystallization kettle, gradually cool down and crystallize until the system temperature is 5-10°C, control the cooling and crystallization time for ...
Embodiment 8
[0084] Embodiment 8 A kind of preparation of isoniazid
[0085] S1, the synthesis of isoniazid
[0086] Dissolve 150kg of isonicotinic acid in 258kg of 40% hydrazine hydrate, distill under reduced pressure to 79-82°C, then change to normal pressure environment, rapidly raise the temperature of the reaction system to 135°C, react for 3 hours, add 193.5kg of purified water to quench The reaction was carried out to obtain the crude product suspension of isoniazid.
[0087] S2, refining of isoniazid
[0088] S2-1. Decolorization: Cool the crude isoniazid suspension obtained in the above step S1. at room temperature for 4 hours, heat the cooled crude suspension to 70-80°C to dissolve, add 2% activated carbon, and stir for 15 minutes. Filter while hot with a filter cloth with a mesh number of 325, quickly transfer the filtrate to the crystallization kettle, gradually cool down and crystallize until the system temperature is 5-10°C, control the cooling and crystallization time for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com