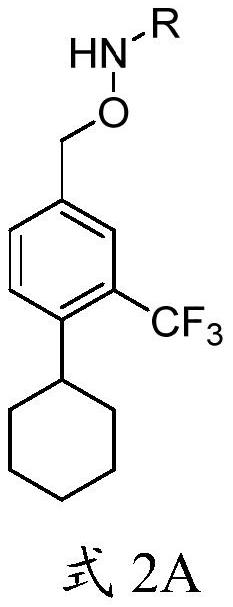
Intermediate of siponimod and synthesis method thereof
A compound and generative technology, applied in the field of organic compound synthesis, can solve the problems of poor bromination selectivity, difficult to purify oily substances, and unsuitable for industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1: Synthesis of N-((4-cyclohexyl-3-(trifluoromethyl)benzyl)oxy)acetamide
[0072] In Example 1, X 1 and x 2 are both bromine and R is Ac.
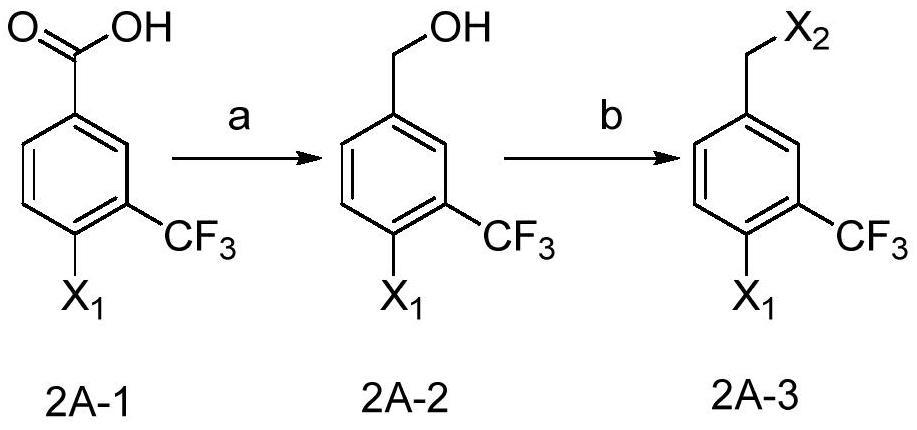
[0073] Step a, synthesis of 4-bromo-3-trifluoromethyl benzyl alcohol
[0074]
[0075] 4-Bromo-3-trifluoromethylbenzoic acid (1.75g, 6.5mmol) was dissolved in THF (5ml), and a THF solution of borane dimethyl sulfide complex (2M, 6.5 ml, 13.0 mmol). After reacting at room temperature for 3 hours, the reaction solution was concentrated to dryness to obtain 1.65 g of a colorless oil with a yield of 99%, which was directly used in the next reaction without further purification.
[0076] Step b, synthesis of 4-bromo-3-trifluoromethylbenzyl bromide
[0077]
[0078] Dissolve 4-bromo-3-trifluoromethylbenzyl alcohol (1.65g, 6.5mmol) obtained in step a in acetic acid (3.3ml), add 33% HBr in acetic acid solution (9ml) at room temperature, add ethyl Anhydride (0.9 g). Then react overnight at room temperature. Subsequent...
Embodiment 2
[0091] Example 2: Synthesis of tert-butyl 4-cyclohexyl-3-trifluoromethylbenzylhydroxycarbamate
[0092] In Example 2, X 1 and x 2 are bromo, and R is Boc.
[0093] Step a and step b are carried out in the same manner as in Example 1.
[0094] Step c, synthesis of ((4-bromo-3-trifluoromethyl)benzyl)oxycarbamate tert-butyl ester
[0095]
[0096] Dissolve tert-butyl N-hydroxycarbamate (0.79g, 5.9mmol) in THF (5ml), add potassium carbonate (0.81g, 5.9mmol) and stir at room temperature for 1h. Subsequently, a solution of 4-bromo-3-trifluoromethylbenzyl bromide (1.88 g, 5.9 mmol) obtained in step b in THF (5 ml) was added and reacted overnight at room temperature. Then the reaction mixture was filtered, and the filtrate was concentrated to dryness to obtain a yellow oil (2.0 g), with a yield of 92%, which was directly used in the next reaction without further purification.
[0097] Step d, synthesis of tert-butyl 4-cyclohex-1-enyl-3-trifluoromethylbenzylhydroxycarbamate ...
Embodiment 3
[0105] Embodiment 3: the synthesis of formula 2A-Ac and 2A-Boc compound
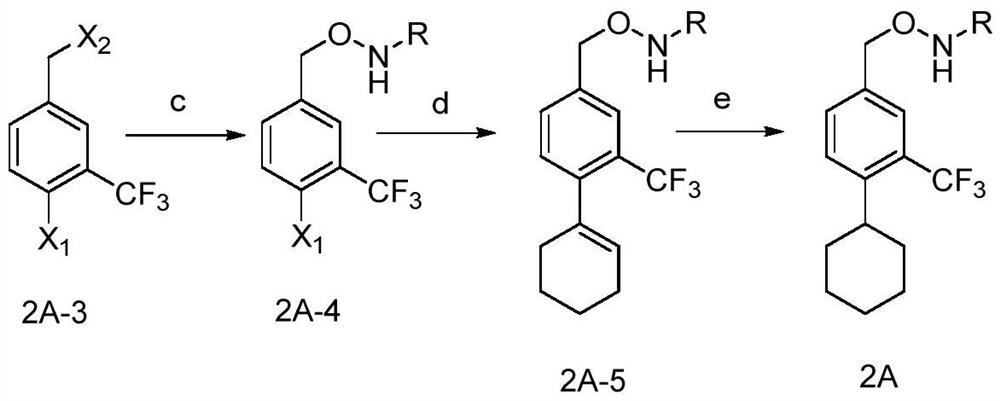
[0106] The present invention also proposes the following specific synthetic routes:
[0107]
[0108] Step a1: Synthesis of 4-bromo-3-trifluoromethylbenzyl bromide (2A-3')
[0109] 4-Bromo-3-trifluoromethyltoluene (3A-1') (0.51g, 2.1mmol) was dissolved in carbon tetrachloride (30ml), and AIBN (0.07g, 0.4mmol) and NBS ( 0.38g, 2.1mmol), heated to 80°C and reacted overnight. After the reaction was completed, DCM (30ml) was added, filtered, and the filtrate was concentrated to dryness. The crude product was mixed with silica gel and passed through the column to obtain 0.2 g of a colorless oil.
[0110] The 4-bromo-3-trifluoromethylbenzyl bromide (2A-3') obtained in the above steps is subsequently carried out from step c to step e in the above Example 1, thereby obtaining the compound of formula 2A-Ac;
[0111] Alternatively, the 4-bromo-3-trifluoromethylbenzyl bromide (2A-3') obtained in the above s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com