Process for the preparation of olefin-olefin alcohol copolymers
The technology of olefin alcohol and copolymer, which is applied in the field of polymer polymer preparation, can solve the problems of scaling, removal of polymer transport solvent, difficulty in granulation, etc., and achieve the effects of not easy scaling, convenient transportation and simple preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
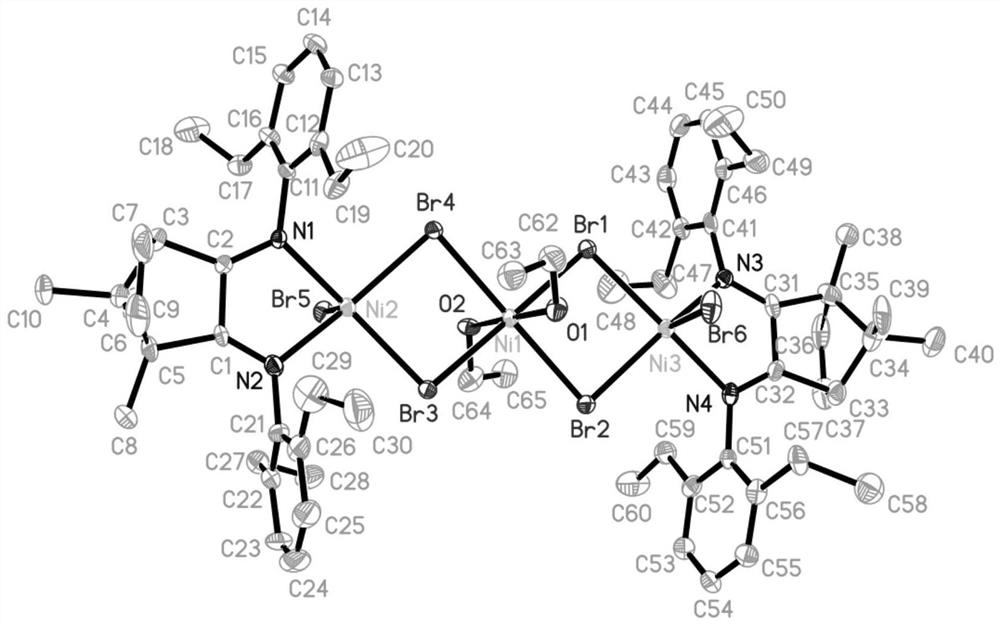
[0117] 1) Ligand L 1 Preparation of:
[0118] Under the protection of nitrogen, 2,6-diethylaniline (2.0mL, 12mmol) was dissolved in 20mL of toluene, and 12mL of trimethylaluminum (1.0M, 12mmol) was added dropwise at room temperature, and the reaction was refluxed for 2 hours, and the system was cooled to room temperature. Camphorquinone (0.831g, 5mmol) was added, and the system was refluxed for 6h. The reaction product was neutralized by aqueous sodium hydroxide solution, extracted and dried with dichloromethane, and the yellow ligand L was obtained by column chromatography. 1 , the yield was 69.2%. 1 H-NMR (CDCl 3 ):δ6.94-6.92(m,6H,C Ar -CH 3 ),2.56-2.51(m,4H,C Ar -CH 3 ),2.36-2.31(m,4H,C Ar -CH 3 ),1.82-1.78(m,4H,CH 2 ),1.54(m,1H),1.24-1.18(m,12H),1.09(s,3H,CH 3 ),0.94(m,6H,CH 3 ).
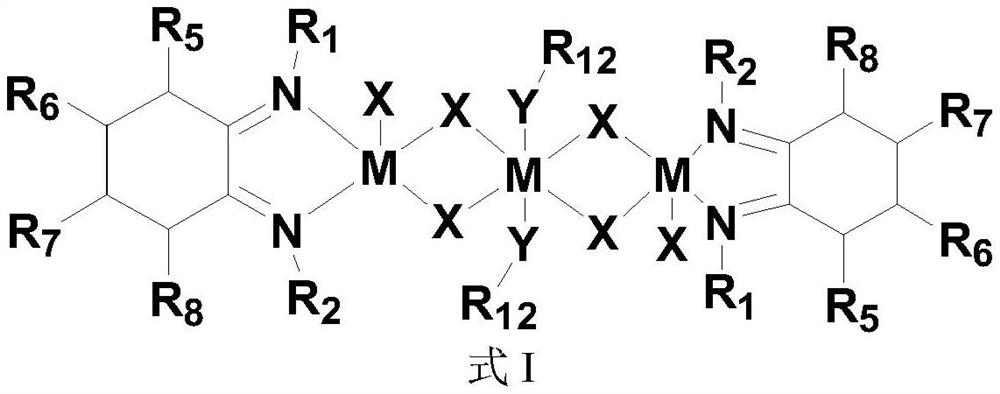
[0119] 2) Complex Ni 1 (R in structural formula III 1 , R 3 is ethyl, R 2 , R 4 -R 7 , R 10 for hydrogen, R 8 , R 9 and R 11 is methyl, R 12 Be ethyl, M is nickel, Y is O...
Embodiment 2
[0124] The present embodiment adopts the prepared catalyst Ni of embodiment 1 1 , the difference from Example 1 is: 2-methyl-2-hydroxyl-7-octene and AlEt 3The consumption is 2 times of embodiment 1.
[0125] Dry the 1L stainless steel polymerization kettle equipped with mechanical stirring at 130 ° C for 6 hours, vacuumize while it is hot and use N 2 Air replacement 3 times. Inject 500 mL of hexane into the polymerization system, and simultaneously add 8.0 mg (5 μmol) of the complex Ni 1 , 30mmol (5.1mL) 2-methyl-2-hydroxy-7-octene, 30mL AlEt 3 (1.0mol / L hexane solution), 6.5mL MAO (1.53mol / L toluene solution), at 30°C, keep 10atm ethylene pressure, and stir for 30min. Finally, it was neutralized with an ethanol solution acidified with 10 wt% hydrochloric acid to obtain a polymer. The polymerization activity and the performance parameters of the polymer are shown in Table 1.
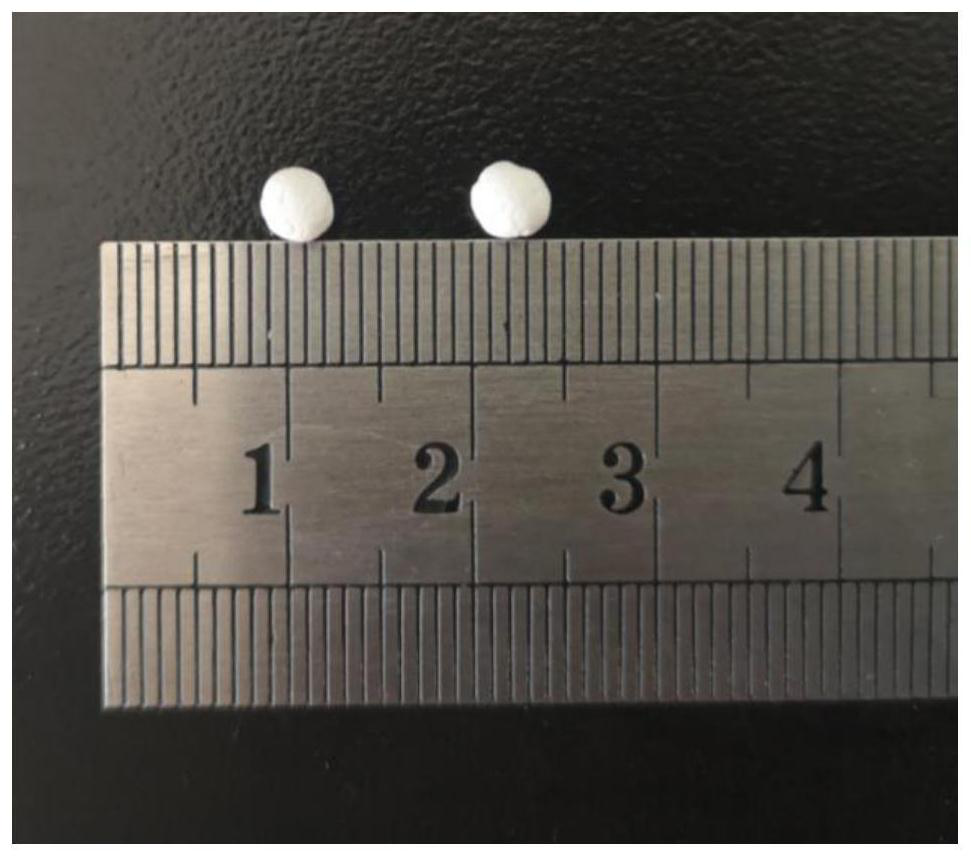
[0126] figure 2 Photographs of spherical and / or quasi-spherical polymers produced in this exa...
Embodiment 3
[0128] The present embodiment adopts the prepared catalyst Ni of embodiment 1 1 , The difference from Example 1 is that the polymerization temperature is 60°C.
[0129] Dry the 1L stainless steel polymerization kettle equipped with mechanical stirring at 130 ° C for 6 hours, vacuumize while it is hot and use N 2 Air replacement 3 times. Inject 500 mL of hexane into the polymerization system, and simultaneously add 8.0 mg (5 μmol) of the complex Ni 1 , 30mmol (5.1mL) 2-methyl-2-hydroxy-7-octene, 30mL AlEt 3 (1.0mol / L hexane solution), 6.5mL MAO (1.53mol / L toluene solution), at 60°C, keep 10atm ethylene pressure, and stir for 30min. Finally, it was neutralized with an ethanol solution acidified with 10 wt% hydrochloric acid to obtain a polymer. The polymerization activity and the performance parameters of the polymer are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com