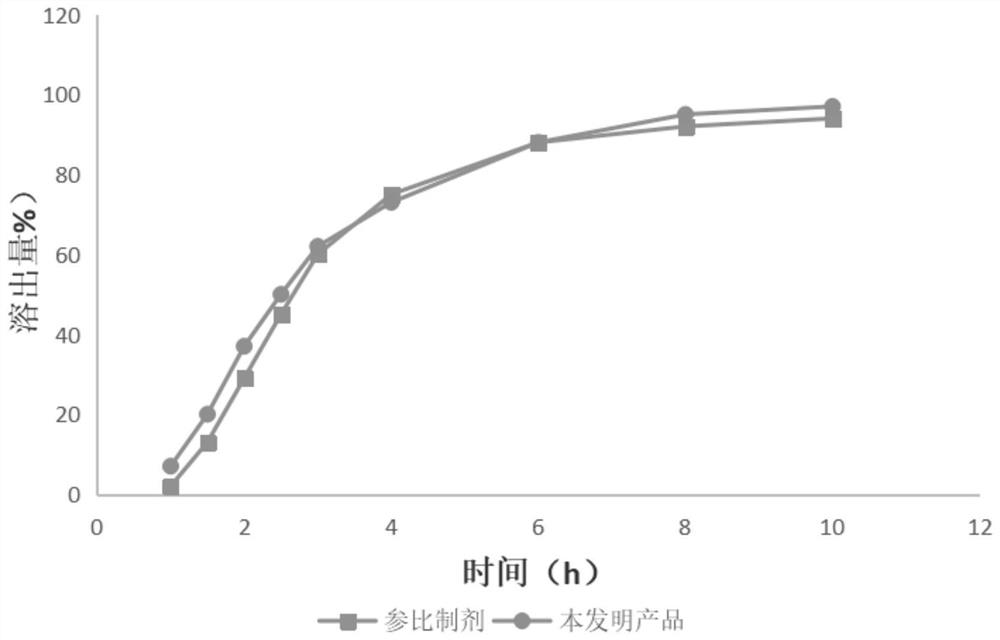
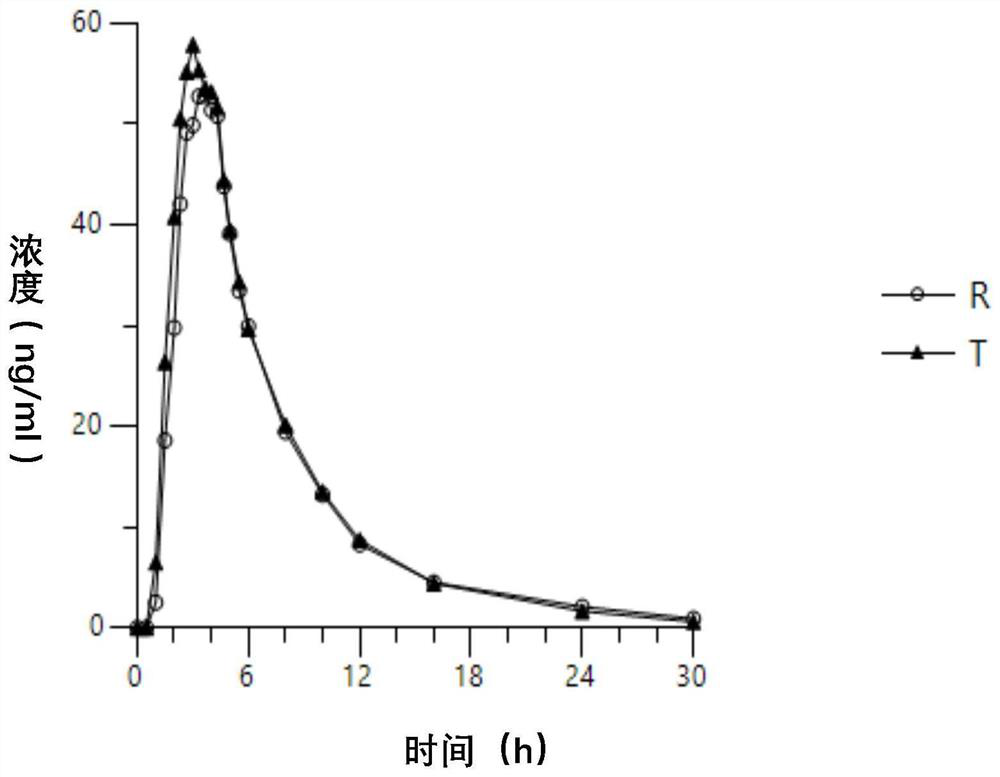
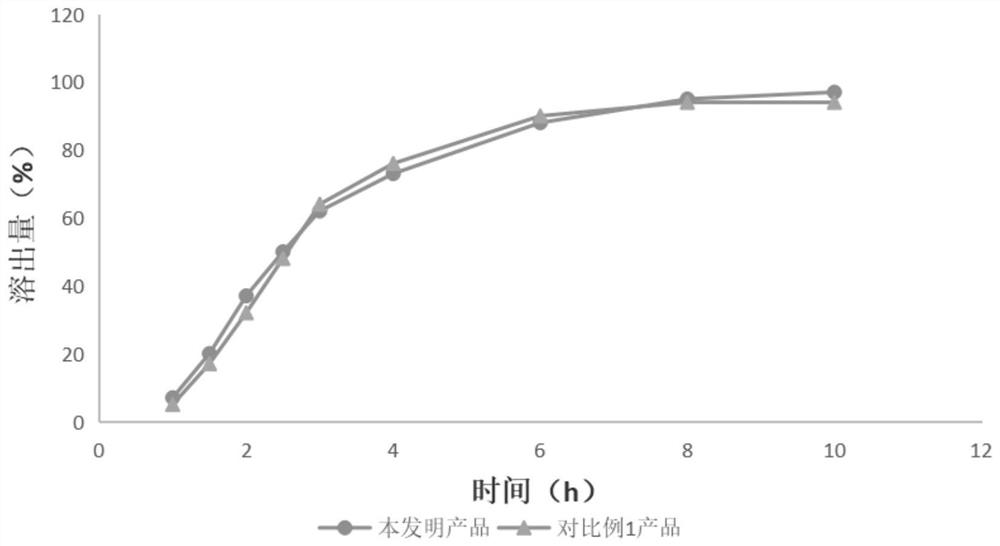
Tofacitinib citrate sustained release tablet
A technology of tofacitinib and citric acid, which is applied in the field of tofacitinib citrate sustained-release tablets and their preparation, can solve related substances, active ingredient content, changes in dissolution, easily affected by external environment, materials Problems such as decreased liquidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0142] The consumption of embodiment 1 osmotic pressure forming agent (sorbitol SI150, sodium chloride) is investigated
[0143] The dosage of osmotic pressure forming agent (sorbitol SI150 and sodium chloride) is screened, and the specific preparation prescription is as follows:
[0144]
[0145] Remarks: *17.77g of tofacitinib citrate is equivalent to 11g of tofacitinib.
[0146] **The perforated tablet is obtained by laser perforation of the sustained-release layer-coated tablet. The perforation diameter is 0.65mm, which has little effect on the tablet weight.
[0147] The preparation method is as follows:
[0148] 1. Pretreatment of raw and auxiliary materials
[0149] Weigh in turn the components of the tablet core: tofacitinib citrate, sorbitol SI150, sodium chloride, hydroxyethylcellulose 250L, hydroxyethylcellulose 250G, copovidone VA64, magnesium stearate, ②Sustained-release layer coating material: cellulose acetate 398-10, hydroxypropyl cellulose EF, ③film coat...
Embodiment 2
[0172] Example 2 Investigation on the dosage ratio of sustained-release materials (hydroxyethyl cellulose 250L, hydroxyethyl cellulose 250G)
[0173] Referring to the prescription 1-1 and its preparation method in Example 1, only the dosage ratio of the sustained-release materials hydroxyethyl cellulose 250L and hydroxyethyl cellulose 250G was adjusted. The specific dosage values and the measured dissolution curve results are shown in Table 2.
[0174] The results show that with the increase of the dosage of hydroxyethyl cellulose 250L and the decrease of the dosage of 250G of hydroxyethyl cellulose, the dissolution rate tends to accelerate.
[0175] Table 2 Influence of composition of sustained-release material on dissolution profile
[0176]
Embodiment 3
[0177] Example 3 Investigation on the dosage ratio of osmotic pressure forming agent and sustained-release material
[0178]With reference to the prescription 1-1 and preparation method of Example 1, adjust the consumption ratio of osmotic pressure forming agent (sorbitol, sodium chloride) and slow-release material (hydroxyethyl cellulose 250L, hydroxyethyl cellulose 250G). The values and dissolution profile results determined are shown in Table 3.
[0179] The results showed that the dosage of osmotic pressure-forming agent was increased and the dosage of slow-release material was decreased in prescription 3-2 compared with prescription 3-1, the dissolution rate of the obtained preparation was accelerated, and the release was basically complete within 6 hours (94% dissolution), resulting in weak release in the later stage. , 6h-10h release only 4%, which is not conducive to maintaining a uniform and stable blood concentration.
[0180] Compared with prescription 3-1, the d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com