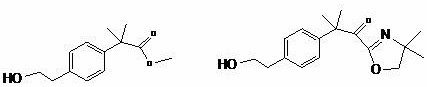
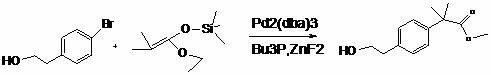Synthetic method of bilastine intermediate
A synthesis method, the technology of bilastine, applied in the field of synthesis of bilastine intermediates, can solve the problems of difficult industrial operation, large pollution in the synthesis process, long synthesis process, etc., to achieve convenient operation and avoid isomers The effect of too much, too little solvent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
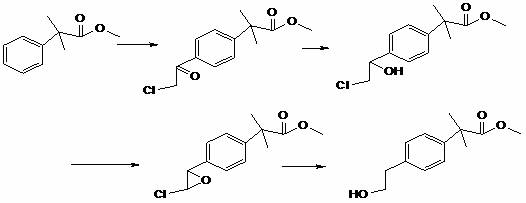
[0041] Example 1 α, the preparation of α-dimethylphenylacetic acid methyl ester
[0042] 200g of benzene, add 40g of hexafluoroisopropanol, stir at room temperature, dropwise add 100g of α-methyl methacrylate, stir at room temperature for 10-15 hours to complete the reaction, recover excess benzene under reduced pressure at 30-40°C, and recover hexafluoroisopropanol Wash with propanol, adjust the water phase to pH=7-9, separate the liquid, dry the organic phase, and distill to obtain 125g of intermediate compound A with a purity of 98% and a yield of 70%.
Embodiment 2
[0043] Example 2 Preparation of α, α-dimethylphenylacetyl diisopropylamine
[0044] 100g of compound A and 200g of diisopropylamine were mixed and stirred at room temperature. After the reaction was complete, the diisopropylamine was recovered by distillation under reduced pressure, washed with water, and dried to obtain 160g of intermediate compound B with a yield of 92%.
Embodiment 3
[0045] Example 3 Preparation of α,α-dimethyl-4-(2-hydroxyethyl)phenylacetyldiisopropylamine
[0046] Cool down 100g of compound B to 0°C, add 50g of ethylene oxide, dropwise add 200g of hexafluoroisopropanol, and stir at 0-5°C to complete the reaction. After the reaction, recover hexafluoroisopropanol under reduced pressure at 30-40°C, wash and dry 106 g of α,α-dimethyl-4-(2-hydroxyethyl)phenylacetyldiisopropylamine was obtained with a purity of 99% and a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com