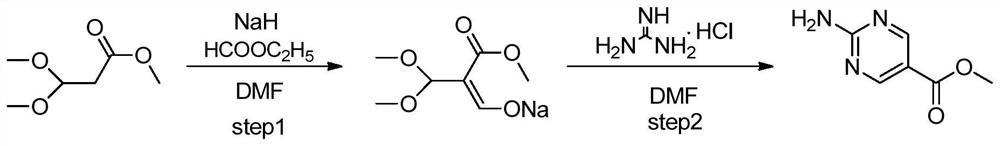
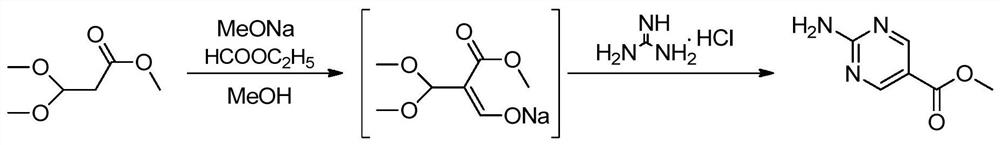
Synthesis method of copanlisib intermediate methyl 2-aminopyrimidine-5-carboxylate
A technology of aminopyrimidine and methyl carboxylate, which is applied in the field of medicine, can solve the problems of excessive waste liquid and waste water, cumbersome reactions, etc., and achieve the effects of less three wastes, simple preparation method and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] Below in conjunction with accompanying drawing, the present invention is described in detail, and the synthetic method chemical equation of this Shen Kupanicil intermediate 2-aminopyrimidine-5-carboxylate is as follows figure 2 Shown, concrete reaction step comprises:
[0018] Step 1: Dissolve sodium methoxide in methanol, cool to room temperature, add ethyl formate, control the temperature in an ice-water bath to less than 30°C, then dropwise add methyl 3,3-dimethoxypropionate, and react at room temperature for 5 hours;
[0019] Step 2: After the reaction, add guanidine hydrochloride and heat up to 50-55°C for 2 hours;
[0020] Step 3: After the reaction is completed, cool down to room temperature, filter with suction, rinse the filter cake with methanol, and dry to obtain the product methyl 2-aminopyrimidine-5-carboxylate.
[0021] The mass ratio of methanol to methyl 3,3-dimethoxypropionate is 10:1~15:1; the molar ratio of sodium methoxide to methyl 3,3-dimethoxypr...
Embodiment 1
[0023] Add 1.0Kg of methanol and sodium methoxide (36.7g, 0.68mol) into a 3L four-necked flask with mechanical stirring and a thermometer, stir to dissolve, cool to room temperature, add ethyl formate (50.4g, 0.68mol), and control the temperature in an ice-water bath The temperature is not higher than 30°C, add 3,3-dimethoxypropionate methyl ester (100.0g, 0.68mol) dropwise, and stir at room temperature for 5 hours after the dropwise addition, after the reaction, add guanidine hydrochloride (64.1g, 0.68mol) , heated to 50-55° C. for 2 hours, cooled to room temperature, suction filtered, washed with methanol, and dried to obtain 86.9 g of methyl 2-aminopyrimidine-5-carboxylate, with a yield of 84%. 1H-NMR (DMSO-d6): δ8.69 (s, 2H), δ7.57 (brs, 2H), δ3.79 (s, 3H).
Embodiment 2
[0025] Add 1.5Kg of methanol and sodium methoxide (36.7g, 0.68mol) into a 3L four-necked flask with mechanical stirring and a thermometer, stir to dissolve, cool to room temperature, add ethyl formate (50.4g, 0.68mol), and control the temperature in an ice-water bath The temperature is not higher than 30°C, add 3,3-dimethoxypropionate methyl ester (100.0g, 0.68mol) dropwise, and stir at room temperature for 5 hours after the dropwise addition, after the reaction, add guanidine hydrochloride (64.1g, 0.68mol) , heated to 50-55° C. for 2 hours, cooled to room temperature, suction filtered, washed with methanol, and dried to obtain 84.1 g of methyl 2-aminopyrimidine-5-carboxylate, with a yield of 81%. 1H-NMR (DMSO-d6): δ8.69 (s, 2H), δ7.57 (brs, 2H), δ3.79 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com