Operating cap material manufacturing process
A production process and technology for surgical caps, which are applied in the manufacture of cap products, caps, rayon, etc., can solve the problems of wound infection of patients and reduced success rate of surgery.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A kind of surgical cap material manufacturing process, specifically comprises the steps:
[0036] Step S1: Add butylene terephthalate, trifluoroacetic acid, and methylene chloride into a stirred tank, and stir for 1 hour at a rotation speed of 500 r / min to obtain a polyester solution;
[0037] Step S2: Add the antibacterial agent and the polyester solution prepared in step S1 into a stirred tank, and after stirring for 5 minutes at a rotating speed of 800 r / min, perform ultrasonic treatment for 1 hour at a frequency of 5 MHz to obtain spinning solution;
[0038] Step S3: Add the spinning solution into the electrospinning apparatus, and spin under the conditions of a pushing rate of 0.001 mm / s, a spinning voltage of 22 kV, and a receiving distance of 5 cm to obtain a surgical cap material.
[0039] Described antibacterial agent is made by following steps:
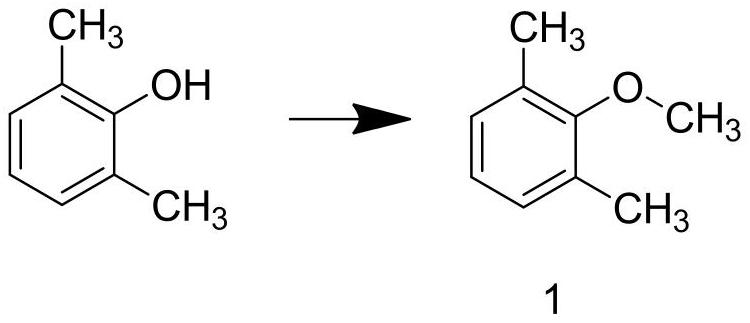
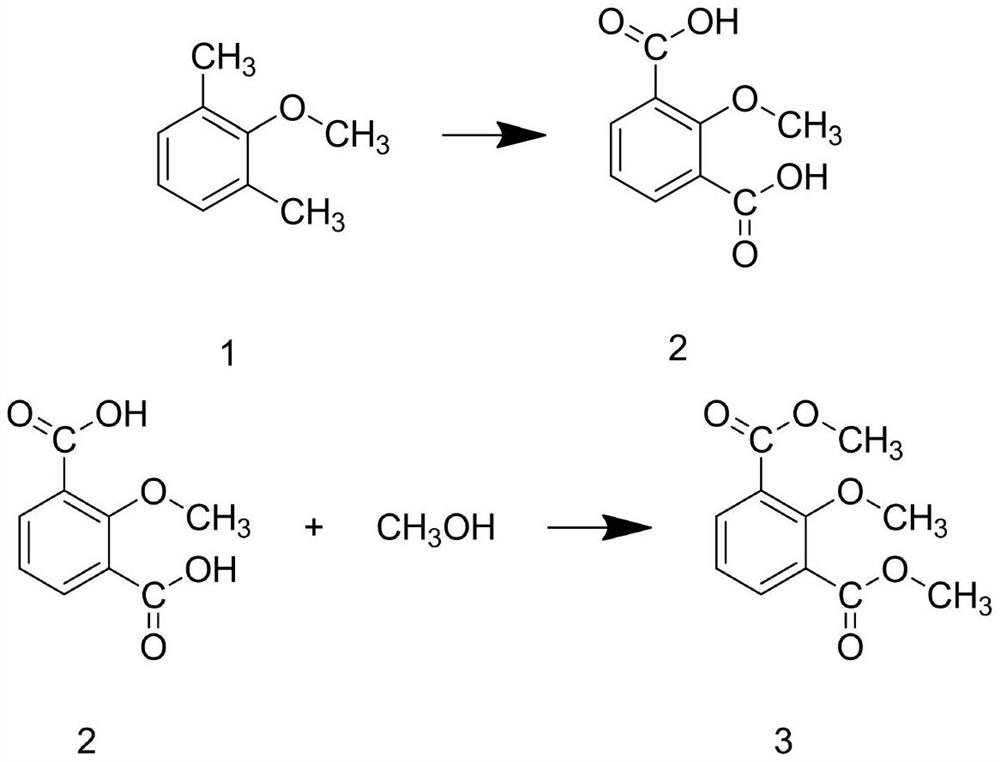
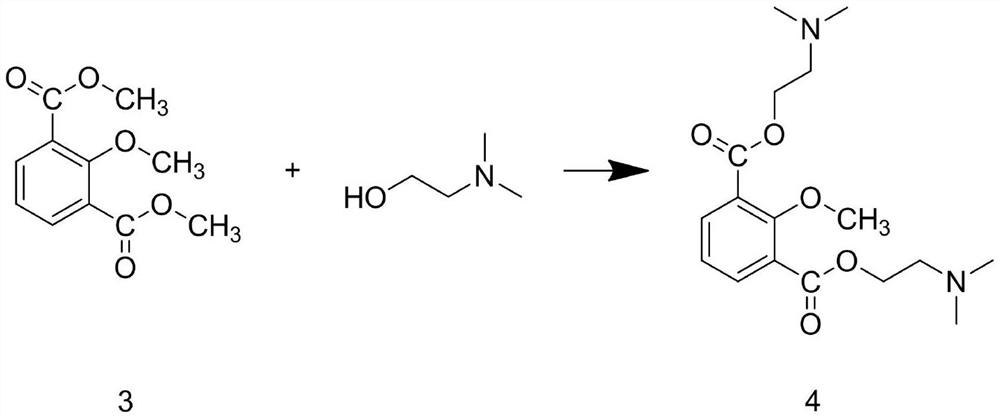
[0040] Step A1: Add 2,6-dimethylphenol and sodium hydroxide solution into the reaction kettle, and stir at a spee...
Embodiment 2
[0046] A kind of surgical cap material manufacturing process, specifically comprises the steps:
[0047] Step S1: adding butanediol terephthalate, trifluoroacetic acid, and methylene chloride into a stirred tank, and stirring for 1.5 hours at a rotating speed of 500 r / min to obtain a polyester solution;
[0048] Step S2: Add the antibacterial agent and the polyester solution prepared in step S1 into a stirred tank, and after stirring for 10 min at a rotating speed of 800 r / min, perform ultrasonic treatment for 1.5 h at a frequency of 5 MHz to prepare spinning solution;
[0049] Step S3: Add the spinning solution into the electrospinning apparatus, and spin under the conditions of a pushing rate of 0.001 mm / s, a spinning voltage of 25 kV, and a receiving distance of 5 cm to obtain a surgical cap material.
[0050] Described antibacterial agent is made by following steps:
[0051] Step A1: Add 2,6-dimethylphenol and sodium hydroxide solution into the reaction kettle, and stir ...
Embodiment 3
[0057] A kind of surgical cap material manufacturing process, specifically comprises the steps:
[0058] Step S1: adding butanediol terephthalate, trifluoroacetic acid, and methylene chloride into a stirred tank, and stirring for 1 hour at a rotating speed of 800 r / min to obtain a polyester solution;
[0059] Step S2: Add the antibacterial agent and the polyester solution prepared in step S1 into a stirred tank, stir for 5 minutes at a rotating speed of 1000 r / min, and then perform ultrasonic treatment for 1 hour at a frequency of 8 MHz to obtain spinning solution;
[0060] Step S3: Add the spinning solution into the electrospinning apparatus, and spin under the conditions of a pushing rate of 0.003 mm / s, a spinning voltage of 22 kV, and a receiving distance of 8 cm to obtain a surgical cap material.
[0061] Described antibacterial agent is made by following steps:
[0062] Step A1: Add 2,6-dimethylphenol and sodium hydroxide solution into the reaction kettle, and stir at a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com