A locally asymmetric small-molecule acceptor material with terminal groups and its application in all-small-molecule organic solar cells
A technology of small molecule acceptor and electron donor materials, applied in the field of solar cells, can solve the problems of low photoelectric conversion efficiency, achieve great application potential and value, high photoelectric conversion efficiency, and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
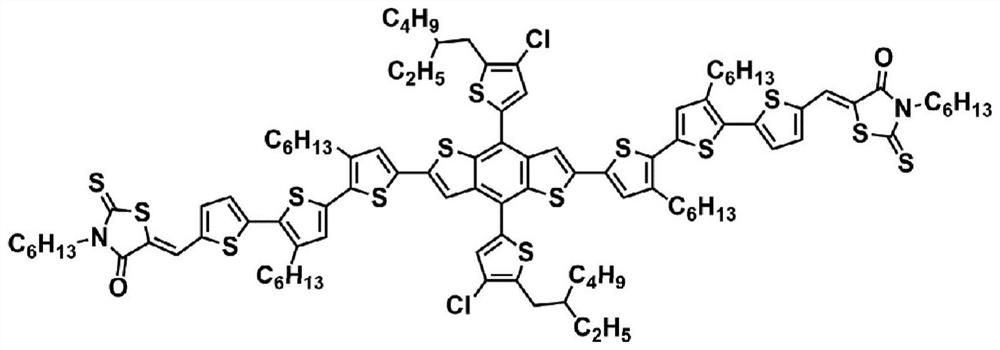
[0050] A forward organic solar cell device was prepared using BTP-FCl-FCl as a small molecule acceptor material. The molecular structure of BTP-FCl-FCl is as follows:
[0051]
[0052] The specific preparation method is as follows:
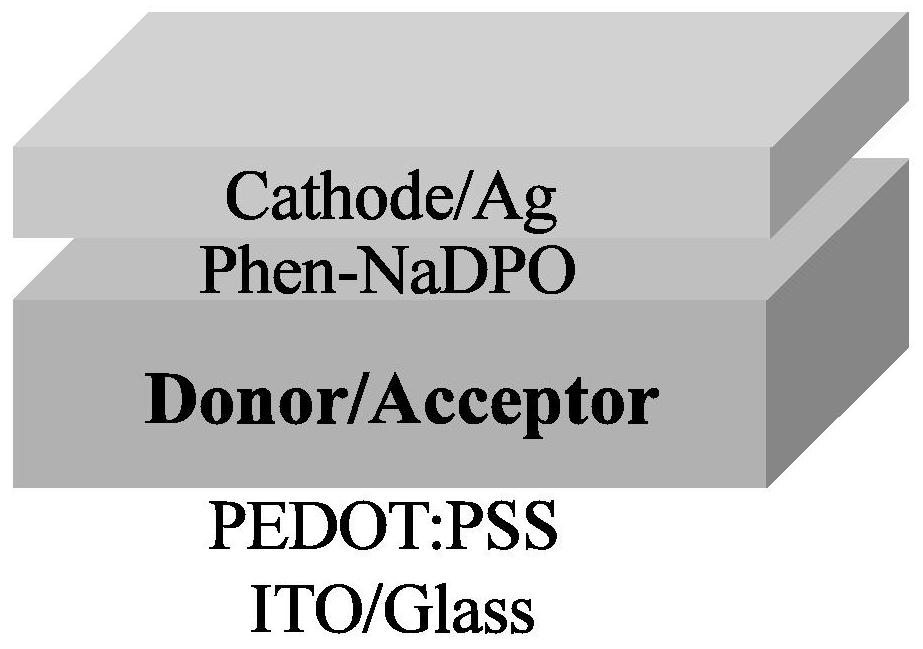
[0053]The substrate composed of transparent glass and transparent conductive electrode ITO was ultrasonically cleaned with cleaning solutions deionized water, acetone and isopropanol, and dried with nitrogen after cleaning; after the substrate was placed in an ozone cleaner for 15 minutes, The hole transport layer material PEDOT:PSS was spin-coated in air (4000rpm, 20s, film thickness 30nm), followed by thermal annealing in air (120°C, 10min), and then the sample was introduced into a glove box filled with nitrogen, On the PEDOT:PSS hole transport layer, the active layer was prepared by spin coating (BTR-Cl:BTP-FCl-FCl=2:1, 17mg / ml, active layer film thickness: ≈110nm), and the obtained active layer The film was subjected to solvent annealing ...
Embodiment 2
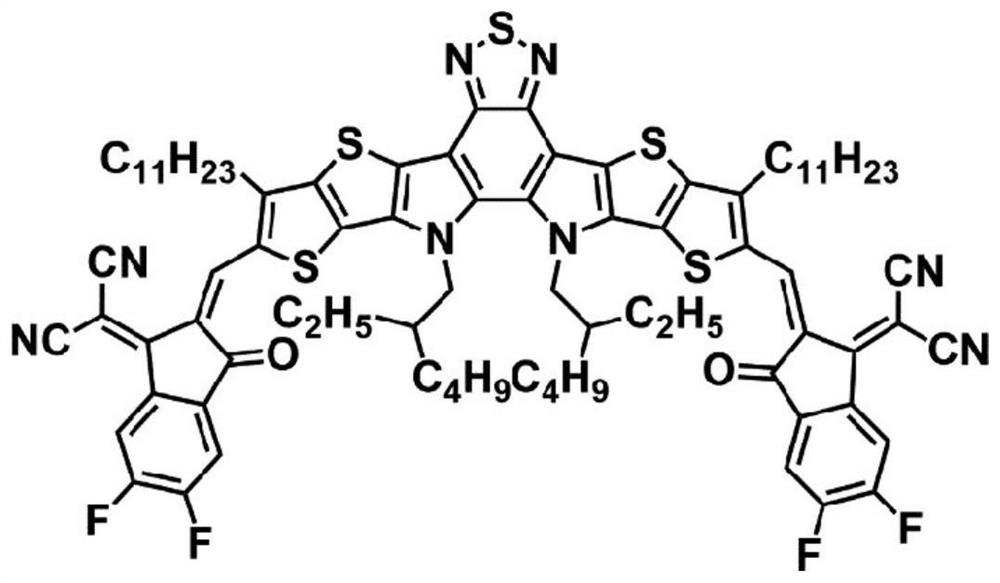
[0055] A forward organic solar cell device was prepared using BTP-FBr-FBr as a small molecule acceptor material. The molecular structure of BTP-FBr-FBr is as follows:
[0056]
[0057] The specific preparation method is as follows:
[0058] The substrate composed of transparent glass and transparent conductive electrode ITO was ultrasonically cleaned with cleaning solution, deionized water, acetone and isopropanol, and dried with nitrogen after cleaning; the substrate was placed in an ozone cleaner for 15 minutes , the hole transport layer material PEDOT:PSS was spin-coated in air (4000rpm, 20s, film thickness 30nm), followed by thermal annealing in air (120°C, 10min), and then the sample was introduced into a glove box filled with nitrogen , on the PEDOT:PSS hole transport layer, the active layer was prepared by spin coating (BTR-Cl:BTP-FBr-FBr=2:1, 17mg / ml, the thickness of the active layer: ≈110nm), and the obtained active layer The thin film was subjected to solvent an...
Embodiment 3
[0060] A forward organic solar cell device was prepared using BTP-FI-FI as a small molecule acceptor material. The molecular structure of BTP-FI-FI is as follows:
[0061]
[0062] The specific preparation method is as follows:
[0063] The substrate composed of transparent glass and transparent conductive electrode ITO was ultrasonically cleaned with cleaning solution, deionized water, acetone and isopropanol, and dried with nitrogen after cleaning; the substrate was placed in an ozone cleaner for 15 minutes , the hole transport layer material PEDOT:PSS was spin-coated in air (4000rpm, 20s, film thickness 30nm), followed by thermal annealing in air (120°C, 10min), and then the sample was introduced into a glove box filled with nitrogen , On the PEDOT:PSS hole transport layer, the active layer was prepared by spin coating (BTR-Cl:BTP-FI-FI=2:1=2:1, 17mg / ml, active layer film thickness: ≈110nm), The obtained active layer film was subjected to solvent annealing treatment (CF...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com