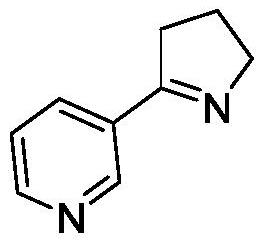
Method for preparing (S)-2-(3-pyridine)-pyrrolidine
A pyrrolidine and pyridine technology, applied in the field of biocatalysis, can solve the problems of increasing the amount of coenzyme NAD, reducing the reaction conversion rate, and high production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the acquisition of highly expressed genetically engineered bacteria
[0038] The whole gene synthesis was completed by General Biosystems (Anhui) Co., Ltd.
[0039] Based on the imine reductase MsIR1 (WP_074958336.1) of the bacterium (Myxococcus fulvus), the codon was optimized in order to enable the gene to be expressed in the E. coli expression host. And Nde I and EcoR I restriction sites were added to both ends of the gene, and constructed into the pET-28a(+) vector to obtain the genetically engineered bacterium M1.
[0040] Transform the prepared recombinant vector into Escherichia coli BL21, Rosetta or Origami by conventional methods to construct a genetically engineered bacterium in which the recombinant imine reductase exists in the bacterium in a soluble form, and screen out the successfully established genetically engineered bacteria, among which Escherichia coli Bacillus BL21 as the host bacterium recombinant bacteria protein expression is relat...
Embodiment 2
[0041] The cultivation of embodiment 2 genetically engineered bacteria and the preparation of crude enzyme liquid
[0042] Pick a single colony on the plate and inoculate it into 5ml of fermentation medium containing corresponding antibiotics, cultivate it for about 15 hours as a seed solution, inoculate it into 600ml of fermentation medium according to the inoculation amount of 1%, and cultivate it on a shaker at 37°C and 200rpm to OD 600 =0.6~0.8, add IPTG with a final concentration of 0.1mM to induce for more than 10h, collect the bacterial cells by centrifuging the culture solution at 8000rpm, and perform high-pressure crushing to obtain the crude enzyme solution of imine reductase.
Embodiment 3
[0043] Example 3 Whole-cell Catalytic Synthesis of (S)-2-(3-Pyridine)-Pyrrolidine Using Imine Reductase
[0044] 10ml phosphate buffer (pH7.5), 30mg / ml bacteria, 2eq glucose, 0.2mg / ml NADP + ,10mg GDH powder, substrate concentration 50mg / ml, react at 28°C, TLC plate to judge the reaction progress. After 12 hours, add saturated sodium hydroxide solution to adjust the pH to above 10, centrifuge to remove denatured protein, extract the supernatant with dichloromethane, dry, spin dry to collect the product, and detect by HPLC.
[0045] serial number pH Conversion rate ee 1 6.0 26.1 99.7 2 6.5 48.9 99.8 3 7.0 84.6 99.7 4 7.5 98.7 99.8 5 8.0 89.1 99.8 6 8.5 80.3 99.7 7 9.0 72.6 99.7 8 9.5 55.8 99.7
[0046] According to the above table, it can be seen that the buffer solution has a higher conversion rate at pH 7.0-9.0, especially at pH 7.5-8.0, the conversion rate effect is very significant.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com