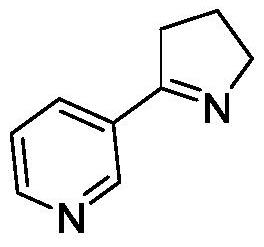
A kind of method for preparing (s)-2-(3-pyridine)-pyrrolidine
A pyrrolidine and pyridine technology, applied in the field of biocatalysis, can solve the problems of increasing the amount of coenzyme NAD, high production cost, and reduced reaction conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Acquisition of high-expressing genetically engineered bacteria
[0038] Whole gene synthesis was performed by General Biosystems (Anhui) Co., Ltd.
[0039] According to the imine reductase MsIR1 (WP_074958336.1) of bacteria (Myxococcus fulvus), it was codon-optimized to enable the gene to be expressed in the E. coli expression host. Nde I and EcoR I restriction sites were added to both ends of the gene to construct into pET-28a(+) vector to obtain genetically engineered bacteria M1.
[0040] The prepared recombinant vector is transformed into Escherichia coli BL21, Rosetta or Origami by conventional methods to construct genetically engineered bacteria in which the recombinant imine reductase exists in the bacteria in a soluble form, and screened out the genetically engineered bacteria that have been established successfully. The recombinant bacteria with Bacillus BL21 as the host bacteria expressed relatively good protein of interest. The engineering bacter...
Embodiment 2
[0041] The cultivation of embodiment 2 genetically engineered bacteria and the preparation of crude enzyme liquid
[0042] Pick a single colony on the plate and inoculate it into 5ml of fermentation medium containing corresponding antibiotics, cultivate for about 15h as seed liquid, inoculate into 600ml of fermentation medium according to 1% of the inoculum, and cultivate at 37°C, 200rpm on a shaking table to OD 600 =0.6-0.8, add IPTG with a final concentration of 0.1 mM for induction for more than 10 hours, centrifuge the culture solution at 8000 rpm to collect bacterial cells, and perform high-pressure crushing to obtain a crude enzyme solution of imine reductase.
Embodiment 3
[0043] Example 3 Whole cell catalytic synthesis of (S)-2-(3-pyridine)-pyrrolidine using imine reductase
[0044] 10ml phosphate buffer (pH7.5), 30mg / ml cells, 2eq glucose, 0.2mg / ml NADP + , 10mg GDH powder, substrate concentration 50mg / ml, 28 ℃ reaction, TLC spot plate to judge the progress of the reaction. After 12 hours, a saturated sodium hydroxide solution was added to adjust the pH value to above 10, and the denatured protein was removed by centrifugation. The supernatant was extracted with dichloromethane, dried, and the product was collected by spin-drying and detected by HPLC.
[0045] serial number pH Conversion rate ee 1 6.0 26.1 99.7 2 6.5 48.9 99.8 3 7.0 84.6 99.7 4 7.5 98.7 99.8 5 8.0 89.1 99.8 6 8.5 80.3 99.7 7 9.0 72.6 99.7 8 9.5 55.8 99.7
[0046] According to the above table, the pH of the buffer solution is 7.0-9.0, and the conversion rate is relatively high, especially when the pH is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com