Antitumor effect potentiator
An anti-tumor and enhancer technology, applied in anti-tumor drugs, antibodies, medical preparations containing active ingredients, etc., can solve the problems of cancer patients who have not seen sufficient treatment effects, and achieve the effect of inhibiting development and inhibiting recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
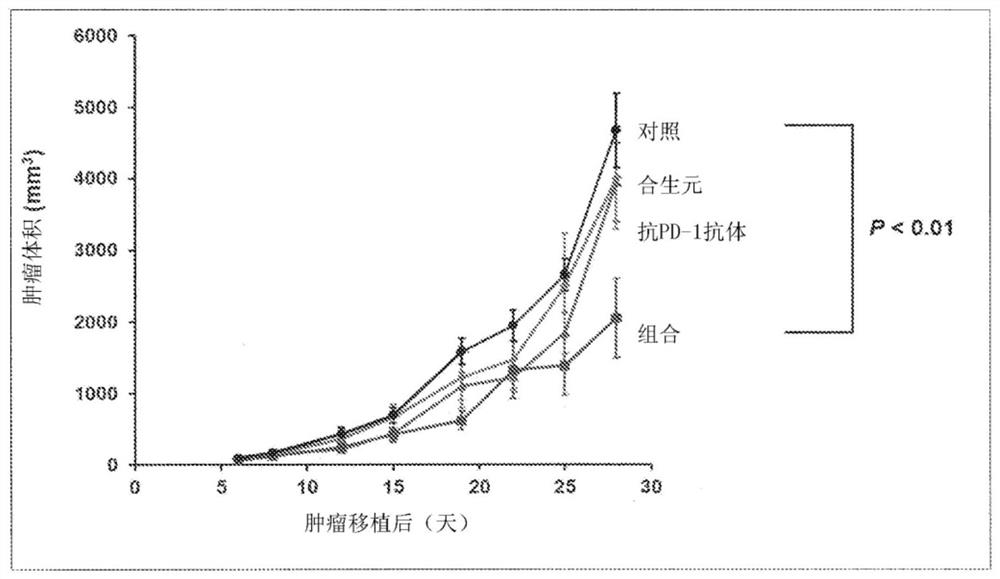
[0085] Example 1 The anti-tumor effect brought about by synbiotics / immune checkpoint inhibitor combined use in vivo
[0086] The mouse colon cancer cell line MC38 cells were transplanted into 7-week-old male C57BL / 6NCrSlc mice to make cell line transplantation model mice. Divided into control (Vehicle) group, synbiotics administration group, anti-PD-1 antibody administration group, synbiotics + anti-PD-1 antibody administration group {n=10 (Vehicle group), 5 (synbiotics administration group) , anti-PD-1 antibody administration group), 6 (synbiotics + anti-PD-1 antibody administration group)}.
[0087] Synbiotics were administered as follows: once a day after transplantation, 0.2 mL of synbiotics / mouse was administered orally every day for 28 days. The anti-PD-1 antibody was administered as follows: from the 7th day of tumor transplantation, 20 mg / kg anti-PD-1 antibody was administered intraperitoneally for the first time, and then administered every 6 days, and 10 mg / kg was a...
Embodiment 2
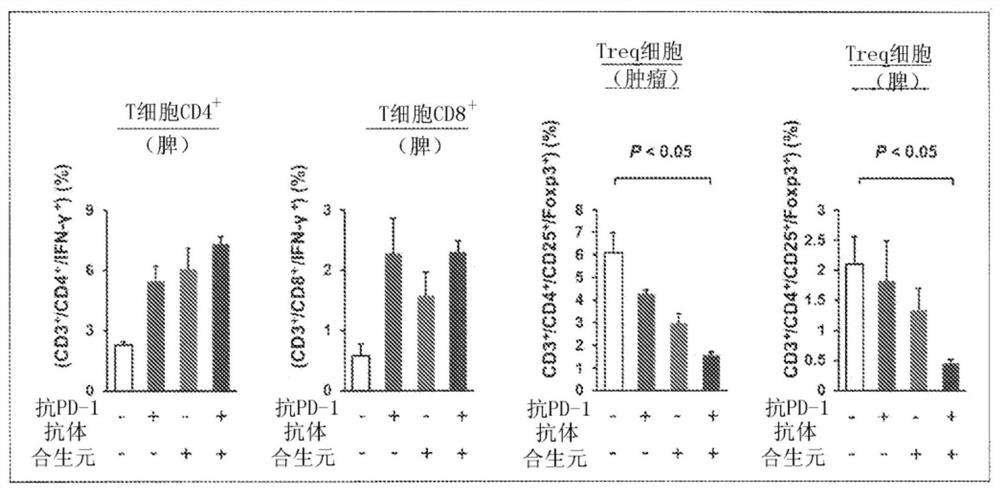
[0091] Example 2 In vivo effects on immune cells caused by combined use of synbiotics / immune checkpoints
[0092] The mouse colon cancer cell line MC38 cells were transplanted into 7-week-old male C57BL / 6NCrSlc mice to make cell line transplantation model mice. Divided into control (Vehicle) group, synbiotics administration group, anti-PD-1 antibody administration group, synbiotics + anti-PD-1 antibody administration group.
[0093] Synbiotics were administered as follows: once a day after transplantation, 0.2 mL of synbiotics / mouse was administered orally every day for 28 days. The anti-PD-1 antibody was administered as follows: from the 7th day of tumor transplantation, 20 mg / kg anti-PD-1 antibody was administered intraperitoneally for the first time, and then administered every 6 days, and 10 mg / kg was administered intraperitoneally for the second to fourth time. medication.
[0094] At the time of dissection, each tissue (spleen, tumor) was recovered, and the cells were ...
Embodiment 3
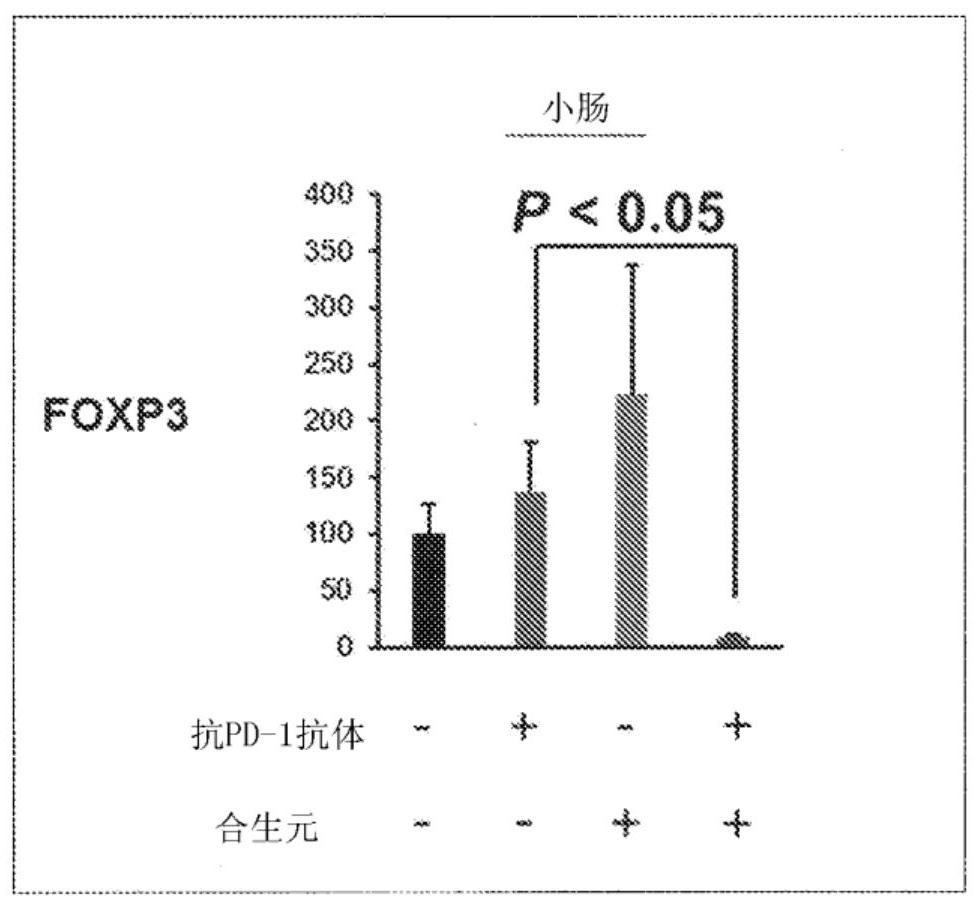
[0096] Example 3 In vivo effects of combined use of synbiotics / immune checkpoint inhibitors on control T cells (Treg) in the small intestine
[0097] The mouse colon cancer cell line MC38 cells were transplanted into 7-week-old male C57BL / 6NCrSlc mice to make cell line transplantation model mice. Divided into control (Vehicle) group, synbiotics administration group, anti-PD-1 antibody administration group, synbiotics + anti-PD-1 antibody administration group {n=10 (Vehicle group), 5 (synbiotics administration group) , anti-PD-1 antibody administration group), 6 (synbiotics + anti-PD-1 antibody administration group)}.
[0098] Synbiotics were administered as follows: once a day after transplantation, 0.2 mL of synbiotics / mouse was administered orally every day for 28 days. The anti-PD-1 antibody was administered as follows: from the 7th day of tumor transplantation, 20 mg / kg anti-PD-1 antibody was administered intraperitoneally for the first time, and then administered every 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com