GalNAc/CpG liposome vaccine with anti-tumor activity as well as preparation method and application of GalNAc/CpG liposome vaccine
A technology of anti-tumor activity and liposomes, which is applied in the direction of anti-tumor drugs, liposome delivery, and medical preparations of non-active ingredients, etc. It can solve the complex synthesis steps of vaccine vectors, clinical application limitations, and difficult qualitative vaccine components and other problems, to achieve good particle size and stability, narrow distribution, and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
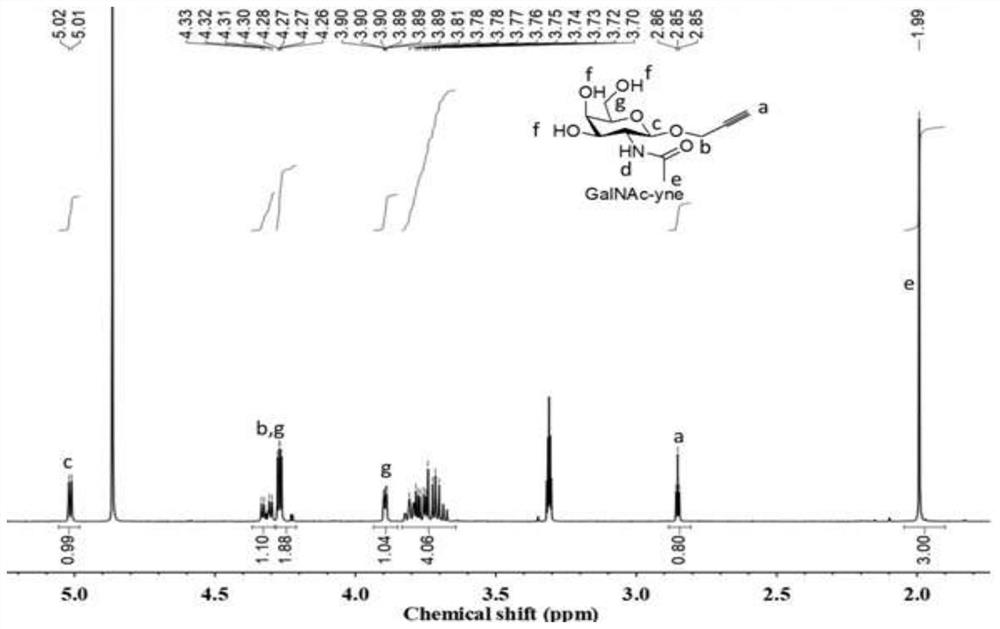
Examples
Embodiment 1
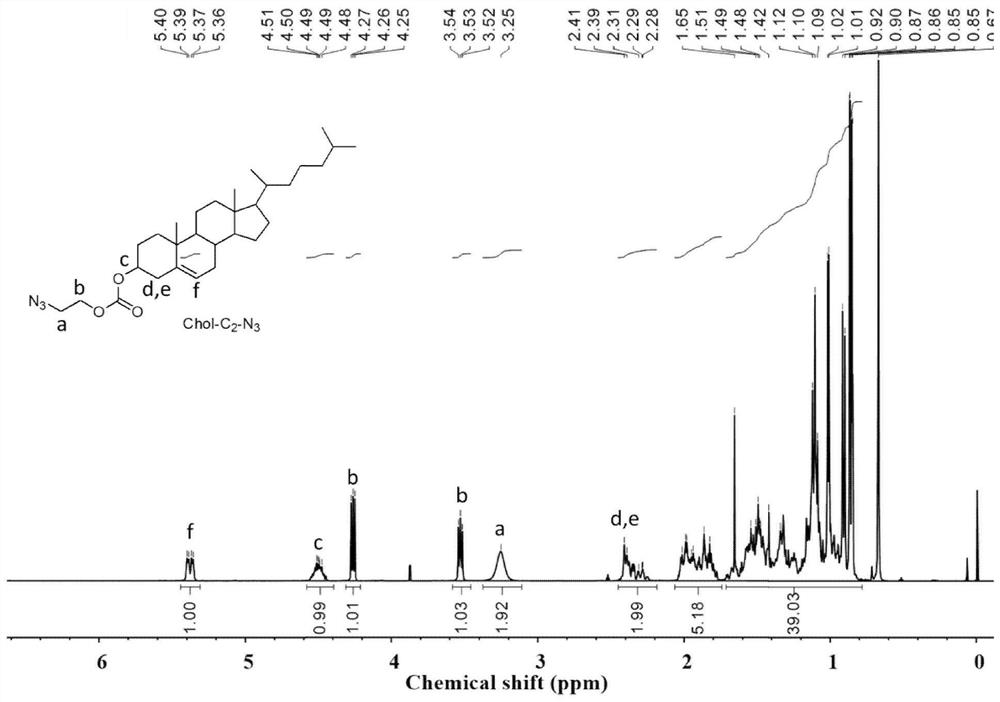
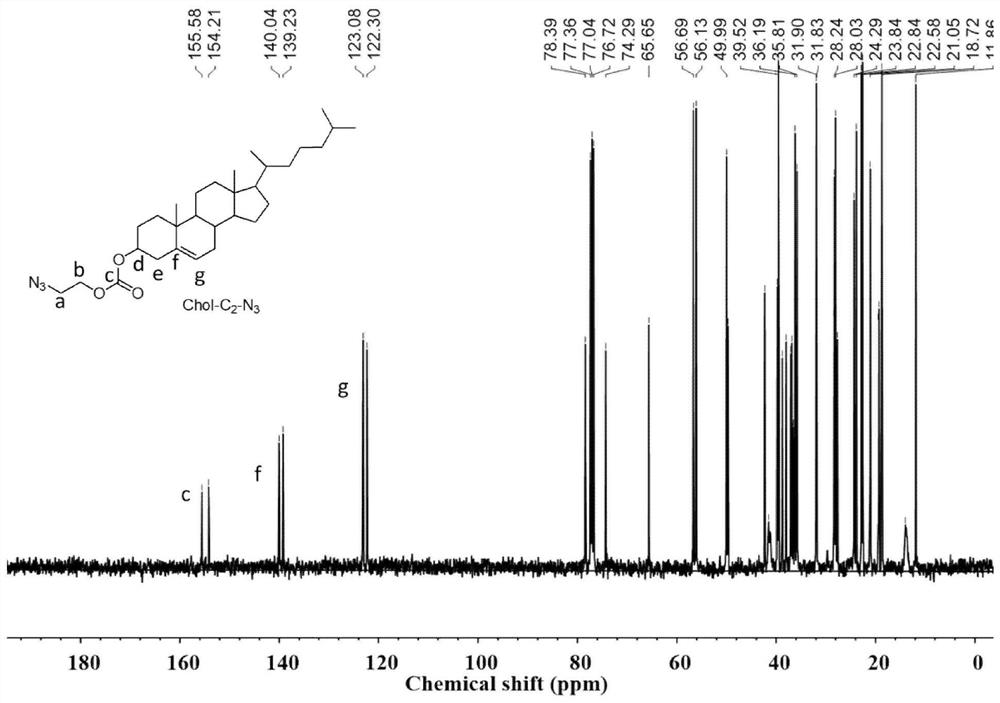
[0045] 1. Chol-C 2 -N 3 Synthesis
[0046] 2-Azidoethanol (0.67g, 7.8mmol) and triethylamine (0.73mL, 5.1mmol) were dissolved in dichloromethane (40mL), and cholesterol formyl chloride Chol-COCl ( 4.1 g, 9.2 mmol), then the solution was returned to room temperature, and the reaction was stirred overnight. After the reaction, it was washed with saturated sodium bicarbonate solution and dried over anhydrous magnesium sulfate. The excess solvent was removed by distillation under reduced pressure, separated by silica gel column chromatography, the mobile phase was petroleum ether / ethyl acetate (20 / 1), Rf=0.5, and the obtained product was used 1 H NMR and 13 The results of C NMR characterization are as follows figure 1 , figure 2 Shown: 1 H NMR (400MHz, CDCl 3 ):δ5.44-5.31(m,1H),4.56-4.43(m,1H),4.31-4.22(m,1H),3.58-3.46(m,1H),3.25(s,2H),2.45-2.22 (m, 2H), 2.06-1.76 (m, 5H), 1.73-0.79 (m, 39H). 13 C NMR (100MHz, CDCl 3 ):δ155.58 140.04123.08 78.39 77.36 77.04 76.72 74.29...
Embodiment 2
[0062] 1. Antigen presentation function of mouse bone marrow-derived dendritic cells BMDCs detected by flow cytometry
[0063] experiment procedure:
[0064] The femur and tibia of BALB / c mice were surgically separated, and both ends of the bone were cut off. The bone marrow cavity was repeatedly flushed with PBS drawn from a 1mL syringe, and the bone marrow tissue was blown away. Red blood cells were removed with ACK lysate to obtain a bone marrow single cell suspension. Adjust the cell density to 1 x 10 6 / mL, cells were cultured with RPMI 1640 complete medium, in which IL-4 (10ng / mL) and GM-CSF (20ng / mL) were added to stimulate the differentiation of bone marrow-derived dendritic cells BMDCs. Fresh media and cytokines were replaced every two and a half volumes. On day 7, the suspended and loosely attached cells were collected as BMDCs. Glycoliposome vaccine (10 μg / mL) stimulated BMDCs for 48 hours, and the cells were collected. With anti-CD40, anti-CD80, anti-CD86 and a...
Embodiment 3
[0080] 1. ELISA identification of the binding ability of glycolipidosome vaccine and Tn antibody
[0081] experiment procedure:
[0082] Glycoliposome vaccine (10 μg / mL) was co-incubated with anti-Tn antibody (Isotype IgM, 5 μg / mL) for 2 hours at 37° C., and the supernatant was collected after centrifugation at 5000 rpm. Using the ELISA method, coat the 96-well plate with Tn antigen overnight, use biotin-labeled anti-IgM antibody as the detection antibody to detect the concentration of anti-Tn antibody, and use the control group 5 μg / mL as the standard to calculate the anti-Tn antibody in the sample Concentration, concrete experimental procedure is as described in embodiment 2 (2). Experimental results such as Figure 11 shown.
[0083] It can be seen from the results in the figure that the GalNAc / CpG liposome vaccine can specifically bind to the Tn antibody, showing good antigenicity.
[0084] 2. ELISA detection of serum antibody titer of immunized mice
[0085] experime...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com