Preparation method of graphene quantum dot sensitized europium-terbium co-doped layered hydroxide and product prepared by the same
A technology of layered hydroxides and graphene quantum dots, applied in the preparation/processing of lanthanide oxides/hydroxides, rare earth metal oxides/hydroxides, rare earth metal compounds, etc., can solve difficult and precise problems Control product structure, disorder of GQD multilayer structure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
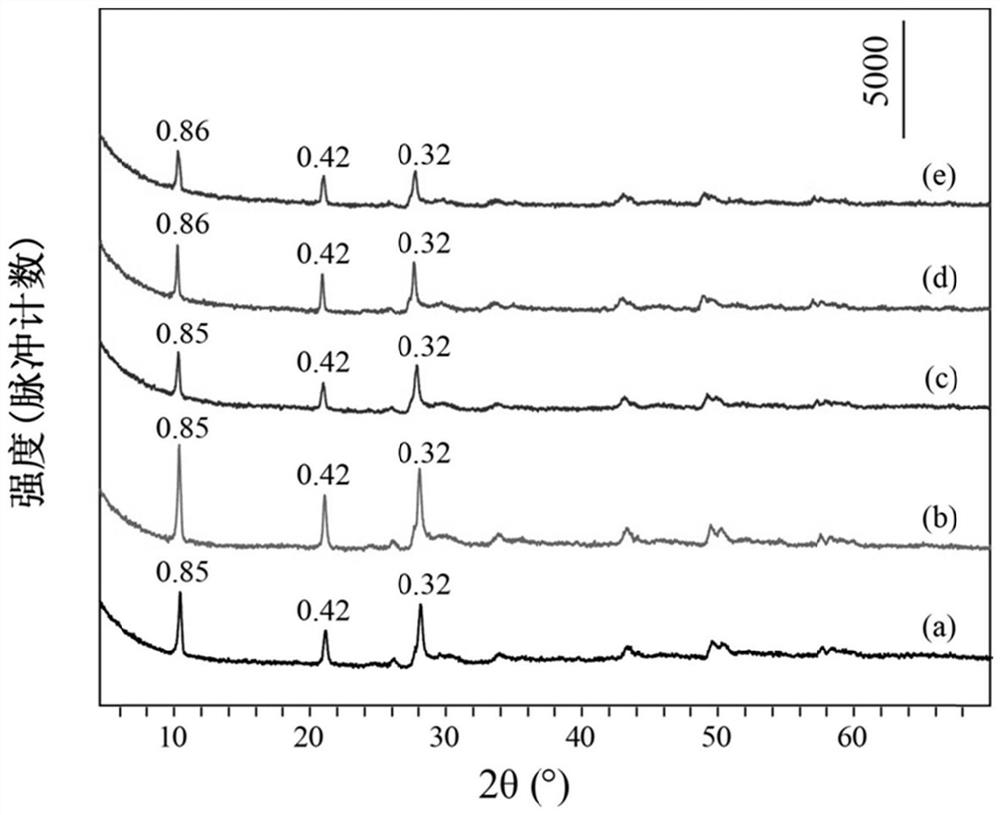
[0035] Take 0.99mmol of Tb (NO 3 ) 3 ·6H 2 O, 0.01mmol of Eu(NO 3 ) 3 ·6H 2 O, 10mmol of NaNO 3 Dissolve 0.5mmol of HMT in 80mL of degassed water, stir evenly, transfer to a 100mL reactor, react at 80°C for 15h, cool to room temperature, wash the precipitate after centrifugation, and dry to obtain NO 3 -LEu 0.01 Tb 0.99 H.
[0036] Take 0.3mmol of NO 3 -LEu 0.01 Tb 0.99 H and 3 mmol Na 3 C 6 h 5 o 7 2H 2 O was dissolved in 80mL of degassed water, stirred evenly, transferred to a 100mL reactor, reacted at 95°C for 20h, cooled to room temperature, washed and dried after centrifugation to obtain CA-LEu 0.01 Tb 0.99 H.
[0037] Weigh 0.1g of CA-LEu 0.01 Tb 0.99 H was dispersed in 80mL exhausted water, 4g of ammonia water was added, stirred evenly, transferred to a 100mL reactor, reacted at 150°C for 10h, cooled to room temperature, centrifuged, washed and dried to obtain GQD-LEu 0.01 Tb 0.99 H.
Embodiment 2
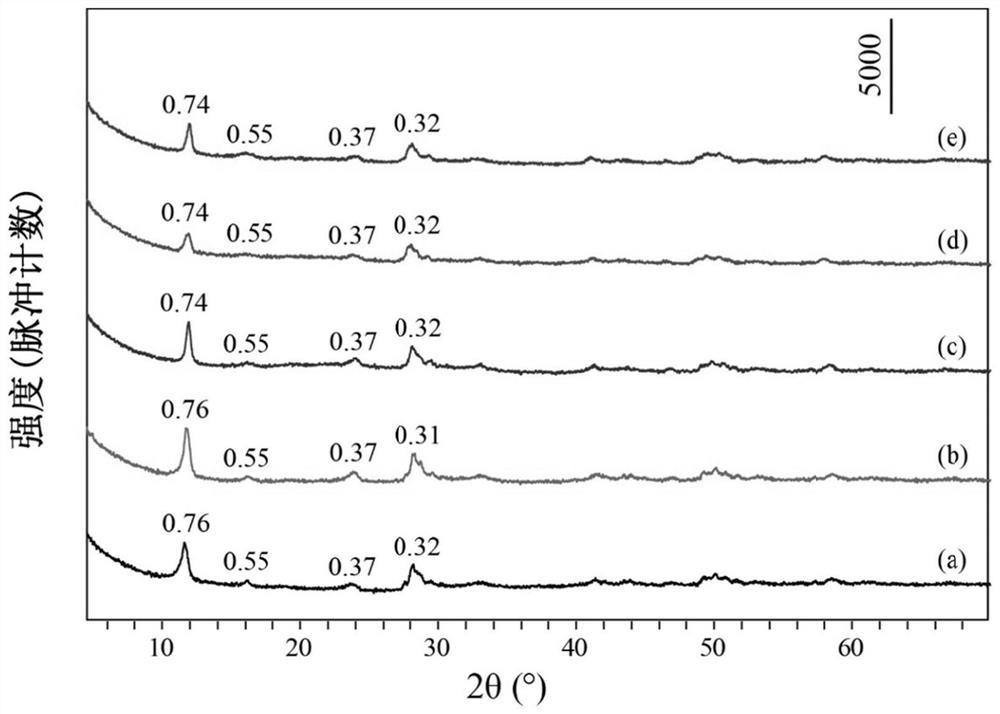
[0039] Take 0.91mmol of Tb(NO 3 ) 3 ·6H 2 O, 0.09mmol of Eu(NO 3 ) 3 ·6H 2 O, 14mmol of NaNO 3 Dissolve 1mmol of HMT in 80mL of degassed water, stir evenly, transfer to a 100mL reactor, react at 90°C for 12h, cool to room temperature, wash the precipitate after centrifugation, and dry to obtain NO 3 -LEu 0.09 Tb 0.91 H.
[0040] Take 0.3mmol of NO 3 -LEu 0.09 Tb 0.91 H and 4.5 mmol Na 3 C 6 h 5 o 7 2H 2 O was dissolved in 80mL of degassed water, stirred evenly, transferred to a 100mL reactor, reacted at 90°C for 24h, cooled to room temperature, washed and dried after centrifugation to obtain CA-LEu 0.09 Tb 0.91 H.
[0041] Weigh 0.1g of CA-LEu 0.09 Tb 0.91 H was dispersed in 80mL of exhausted water, 5g of ammonia water was added, stirred evenly, transferred to a 100mL reactor, reacted at 180°C for 8h, cooled to room temperature, centrifuged, washed and dried to obtain GQD-LEu 0.09 Tb 0.91 H.
Embodiment 3
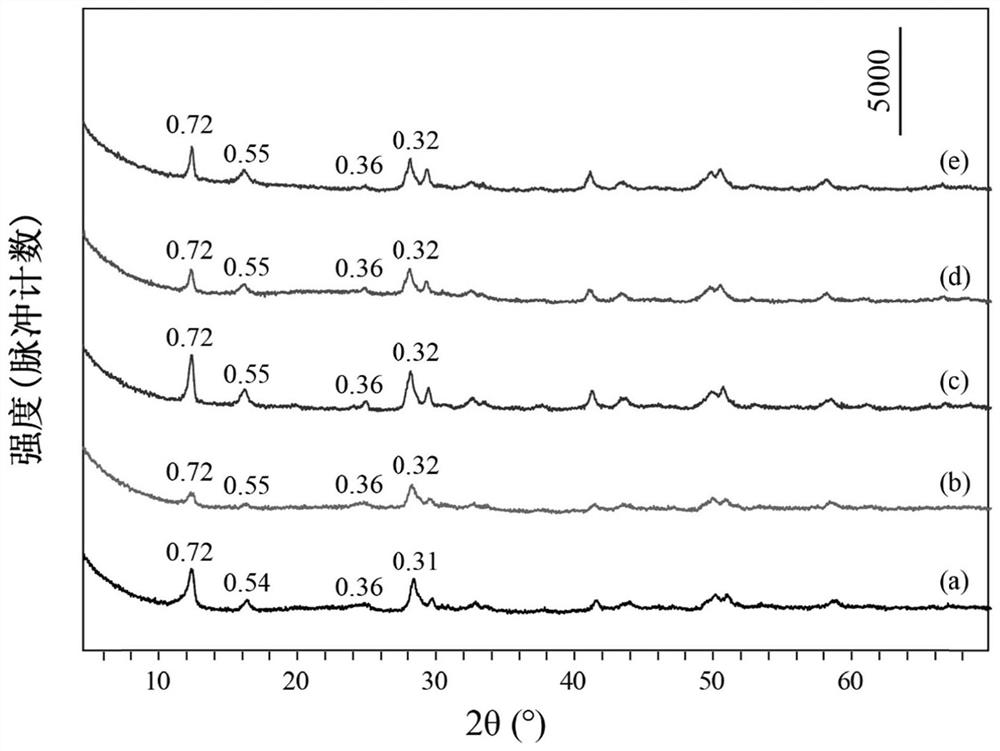
[0043] Take 0.5mmol of Tb(NO 3 ) 3 ·6H 2 O, 0.5mmol of Eu(NO 3 ) 3 ·6H 2 O, 15mmol of NaNO 3 Dissolve 1.5mmol of HMT in 80mL of degassed water, stir evenly, transfer to a 100mL reactor, react at 80°C for 15h, cool to room temperature, wash the precipitate after centrifugation, and dry to obtain NO 3 -LEu 0.5 Tb 0.5 H.
[0044] Take 0.3mmol of NO 3 -LEu 0.5 Tb 0.5 H and 4 mmol Na 3 C 6 h 5 o 7 2H 2 O was dissolved in 80mL of degassed water, stirred evenly, transferred to a 100mL reactor, reacted at 95°C for 20h, cooled to room temperature, washed and dried after centrifugation to obtain CA-LEu 0.5 Tb 0.5 H.
[0045] Weigh 0.1g of CA-LEu 0.5 Tb 0.5 H was dispersed in 80mL of exhaust water, 4.8g of ammonia water was added, stirred evenly, transferred to a 100mL reactor, reacted at 150°C for 10h, cooled to room temperature, centrifuged, washed and dried to obtain GQD-LEu 0.5 Tb 0.5 H.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com