Method for measuring in-vitro dissolution rate of nifedipine controlled release tablets
A determination method and technology of nifedipine, which can be used in measuring devices, testing pharmaceutical preparations, instruments, etc., can solve problems such as inability to correlate with actual situations and inability to fully simulate the drug dissolution/absorption process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
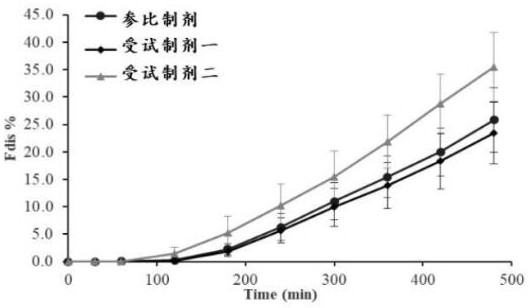
[0049] A kind of assay method of in vitro dissolution rate of nifedipine controlled-release tablet adopts differential dissolution apparatus to measure, and concrete steps are:
[0050] 1) The temperature is controlled at (37±0.5) ℃, the pH 6.8 phosphate buffer solution of 0.2% SDS of the dissolution medium enters the dissolution vessel from the liquid inlet at a speed of 8 mL / min, and the nifedipine controlled-release tablets in the dissolution vessel Carry out stripping, the stripping solution that dissolves nifedipine is exported to sampling device at the speed of 8 mL / min, respectively in 30, 60, 120, 180, 240, 300, 360, 420, 480 min to the sample in the sampling device. collection;
[0051] 2) Precisely measure 20 μL of the eluate, inject it into the high-performance liquid chromatograph, record the chromatogram, calculate the corresponding concentration of nifedipine, and obtain the concentration of nifedipine and the dissolution time based on the relationship between th...
Embodiment 2
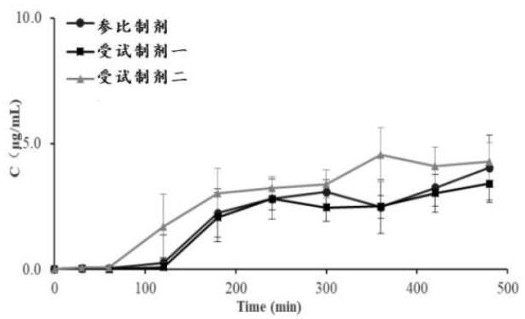
[0056] A kind of assay method of in vitro dissolution rate of nifedipine controlled-release tablet adopts differential dissolution apparatus to measure, and concrete steps are:
[0057] 1) The temperature is controlled at (37±0.5) ℃, the pH 6.8 phosphate buffer solution of 0.2% SDS of the dissolution medium enters the dissolution vessel from the liquid inlet at a speed of 8 mL / min, and the nifedipine controlled-release tablets in the dissolution vessel Carry out stripping, the stripping solution that dissolves nifedipine is exported to sampling device at the speed of 8 mL / min, respectively in 30, 60, 120, 180, 240, 300, 360, 420, 480 min to the sample in the sampling device. collection;
[0058] 2) Precisely measure 20 μL of the eluate, inject it into the high-performance liquid chromatograph, record the chromatogram, calculate the corresponding concentration of nifedipine, and obtain the concentration of nifedipine and the dissolution time based on the relationship between th...
Embodiment 3
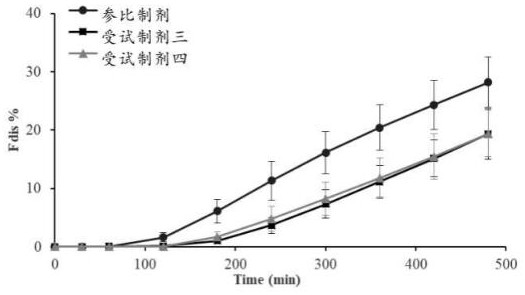
[0062] p-nifedipine controlled-release tablets (test preparation T (reference preparation 3 in Example 2), specification: 30 mg; reference preparation, trade name: Adalat ® , specification: 30mg) to conduct absorption kinetics study, to preliminarily evaluate the consistency of absorption kinetics between the test preparation and the reference preparation.
[0063] The postprandial trial plans to enroll 12 subjects, and adopts a randomized, open, two-drug, two-period (T-R, R-T), double-crossover self-controlled trial design, with a washout period of 7 days between cycles, and a single oral dose per cycle 1 nifedipine controlled-release tablet (test preparation T, strength: 30 mg) or 1 nifedipine controlled-release tablet (reference preparation, trade name: Adalat ® , specification: 30mg).
[0064] The postprandial test blood collection time is designed as: before administration (0h) and after administration 2h, 3h, 4h, 5h, 6h, 7h, 8h, 9h, 10h, 11h, 12h, 14h, 16h, 18h, 20h, 24...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com