Primer group for detecting mycoplasma pneumoniae, kit and method
A Mycoplasma pneumoniae and kit technology, applied in the fields of in vitro diagnosis and detection, and molecular biology, can solve the problems of high requirements for instrument platforms and operators, complex nucleic acid extraction and detection operations, limited use scenarios, etc., and achieve simple and enhanced identification. Convenience, the effect of improving the detection speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1: LAMP method amplifies MP sequence
[0081] (1) Amplification reaction system:
[0082] 1.0mmol / L dNTP;
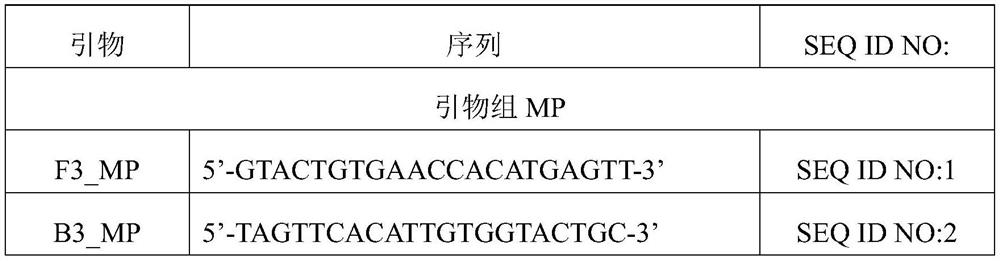
[0083] 0.2μmol / L MP outer primer pair;
[0084] 1.6μmol / L MP inner primer pair;
[0085] 0.4μmol / L MP loop primer pair;
[0086] 0.5U / μL Bst DNA polymerase;
[0087] 20mmol / L Tris-HCl (pH 8.8);
[0088] 10mmol / L KCl;
[0089] 10mmol / L (NH 4 ) 2 SO 4 ;
[0090] 0.1% Triton X-100;
[0091] 6mmol / L MgSO 4 ;
[0092] 0.5mol / L betaine;
[0093] 100X diluted SYBR Green.
[0094] (2) Amplification conditions: placed in a constant temperature nucleic acid amplification analyzer, amplified at 65°C for 20 minutes.
Embodiment 2
[0095] Embodiment 2: Detection of LAMP reaction sensitivity
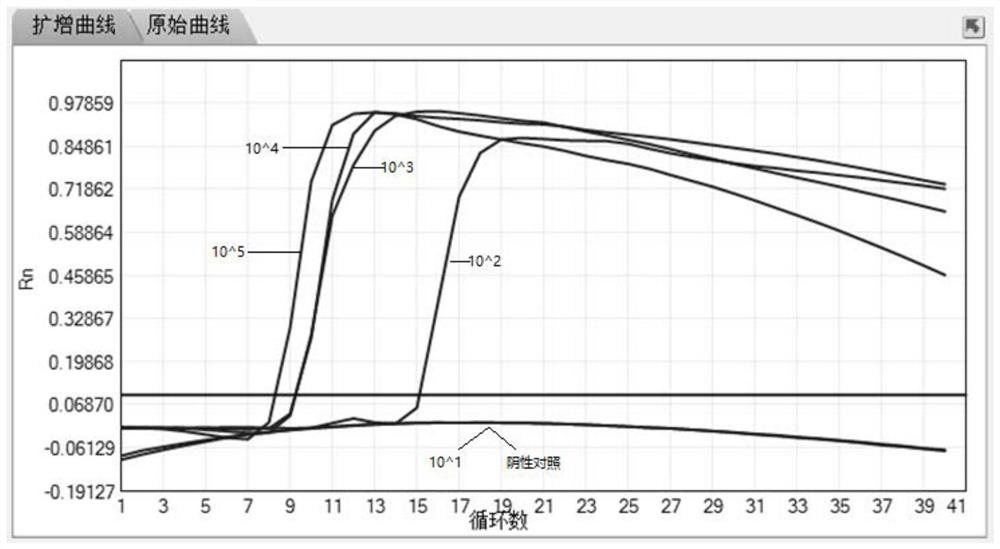
[0096] At present, the mainstream molecular diagnostic products on the market are mostly based on QPCR. Although it is generally believed that the sensitivity performance is good (100-500copies / reaction), it is limited by the need for expensive variable temperature fluorescent quantitative PCR instruments and the slow heating and cooling speed of PCR cycles. And other disadvantages, the promotion has been limited. In contrast, the characteristics of isothermal LAMP technology just make up for the disadvantages of QPCR technology, but the literature reports and the sensitivity performance of LAMP products on the market are uneven. System optimization ensures that LAMP has high detection sensitivity.
[0097] Such as figure 1 , Table 2. The S-shaped curve in the figure is the fluorescence curve of the LAMP amplification experiment. The S-shaped curve means that the fluorescent signal increases. The essential princip...
Embodiment 3
[0101] Embodiment 3: detection of time-consuming reaction
[0102] Such as figure 1 , Table 2, and shown in Table 3, the primer set selected by the present invention can complete the LAMP amplification reaction within 20 minutes, which is much better than the time-consuming of 30-60 minutes of LAMP / mainstream QPCR products reported in the literature and on the market.
[0103] Table 3: Comparison of onset times of different MP primer sets
[0104]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com