Synthesis method of castor oil acyl sulfamate ampholytic surfactant
A technology of oleoyl sulfamate and surfactants, which is applied in the synthesis field of castor oil acyl sulfamate amphoteric surfactants, can solve the problem of poor surface/interface activity, increased purification costs, and poor water solubility and other problems, to achieve the effect of excellent compatibility, high surface/interface activity, and good interface activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] After castor oil was washed 3 times with hot saturated brine, 50 g of treated castor oil was poured into a reaction flask with a stirrer and a temperature sensor, and 30 g of 20% NaOH aqueous solution was added dropwise at room temperature, and the temperature was raised to 65° C. for reaction After 3 hours, solution A was obtained; solution A was washed twice with saturated brine to obtain product B.
[0021] 29mL of diethylenetriamine and 15mL of xylene are contained in a reaction flask equipped with a stirrer, a temperature sensor and a water separator. Add 18.8g of product B in batches at 80°C. 172°C, react until the acid value is 8.62g / g, then diethylenetriamine is distilled off under reduced pressure to obtain product C.
[0022] At room temperature, add 18.4g of product C to a reaction flask containing 42mL of 30% aqueous sodium chloroethylsulfonate solution, stir well, then raise the temperature to 80°C to start the reaction, and adjust the pH of the system with...
Embodiment 2
[0024] After castor oil was washed 3 times with hot saturated brine, 198g of treated castor oil was poured into a reaction flask with a stirrer and a temperature sensor, and 102g of 20% NaOH aqueous solution was added dropwise at room temperature, and the temperature was raised to 72°C for reaction After 3.2 hours, solution A was obtained; solution A was washed three times with saturated brine to obtain product B.
[0025] 116mL of diethylenetriamine and 52mL of a mixture of toluene and xylene (mixed at a volume ratio of 2:8) were contained in a reaction flask with a stirrer, a temperature sensor and a water separator, and 76.4g of product B was mixed in batches at 85°C After adding nitrogen, stir evenly, slowly raise the temperature to 166°C, react until the acid value is 9.74g / g, and then diethylenetriamine is distilled off under reduced pressure to obtain product C.
[0026] At room temperature, add 71.6g of product C into a reaction flask containing 162mL of 32% 2-hydroxy-...
Embodiment 3
[0028] The surface / interface activity, water solubility and compatibility with other surfactants of the products synthesized in Example 1 and Example 2 were studied, see Table 1 and Table 2 below.
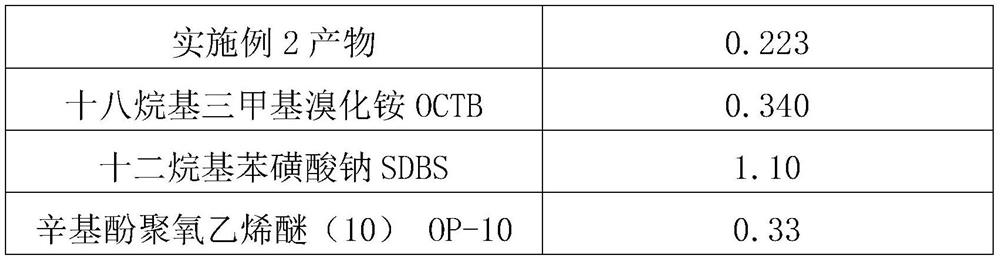
[0029] Table 1 critical micelle concentration (cmc)
[0030]
[0031]
[0032] Wherein: the critical micelle concentration of embodiment 1, embodiment 2 product adopts tension method and conductometric method to estimate, get the average value of both; Reference for the critical micelle concentration value of octylphenol ethoxylate (10): Rosen M.J.Surfactants and Interfacial Phenomena (3th edition)[M].JohnWiley&Sons.Inc.,2004.
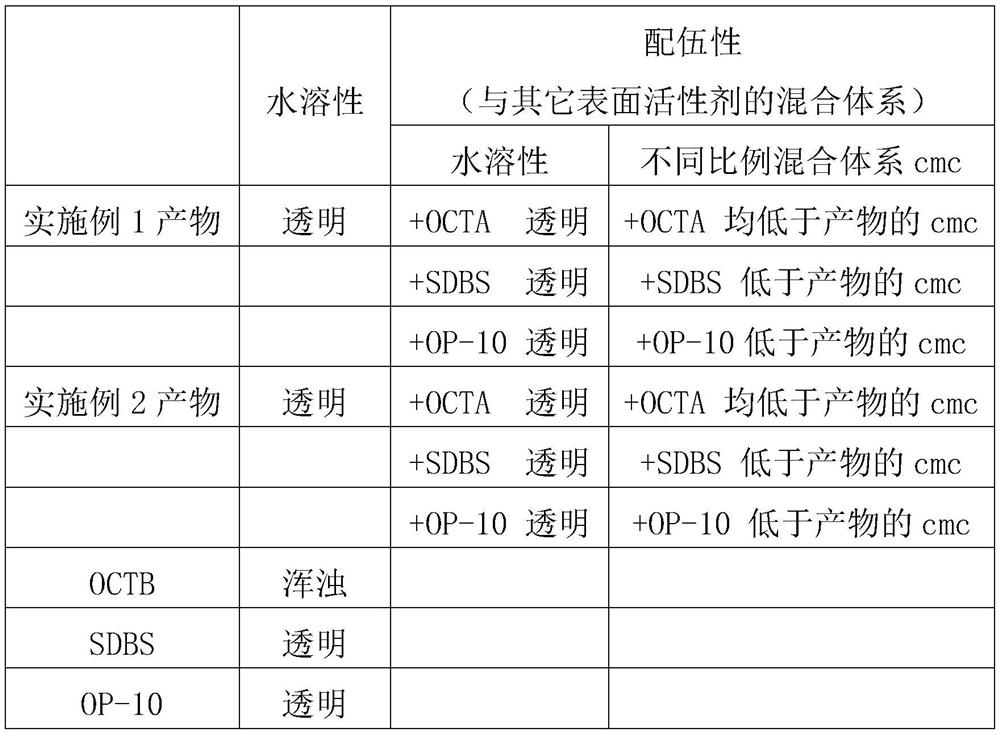
[0033] Table 2 Water Solubility and Compatibility
[0034]
[0035] Wherein: water solubility is evaluated by the appearance of 1 wt% aqueous solution (22°C).
[0036] From the data in Table 1, it can be known that the synthesized product of the present invention shows better surface / interface activity.
[0037] Table 2 shows that the product sy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com