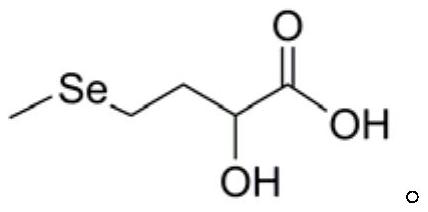
Preparation method of DL-hydroxy selenomethionine
A technology of hydroxyselenomethionine and hydroxyl, which is applied in the field of preparation of organic compounds, and can solve problems such as poor stability, difficulty in obtaining, and high cost of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example
[0015] The present invention will be described in detail below using examples. However, the present invention is not limited to the forms shown in the examples, and the specific embodiments can be variously changed within the range described in the specific embodiments of the present invention.
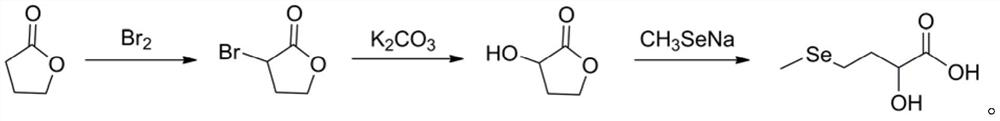
[0016] 1 Preparation of α-bromo-γ-butyrolactone
[0017] Add 2.2ml of phosphorus tribromide to 100g of γ-butyrolactone, heat to 100°C under the protection of nitrogen, and slowly add 176.4g of bromine dropwise. After the dropwise addition, continue to keep warm and stir for 4 hours. After the reaction, concentrate under reduced pressure to remove the low boiling point. 172.5 g of the crude product of α-bromo-γ-butyrolactone was obtained, which was directly used in the next reaction.
[0018] 2 Preparation of α-hydroxy-γ-butyrolactone
[0019] Dissolve 217g of potassium carbonate in 1L of deionized water, heat to reflux, slowly add dropwise the crude product of 172.5 g of α-bromo-γ-b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com