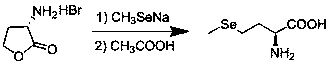Method for preparing L-selenomethionine
A technology for selenomethionine and methionine, which is applied in the field of preparation of L-selenomethionine, can solve the problems of long synthesis route, unfavorable cost control of L-selenomethionine, and high purchase cost, achieves low cost, is beneficial to labor protection and Environment, preparation of simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The invention provides a method for preparing L-selenomethionine. Through research, it is found that the dimethyl sulfide group after the reaction of L-methionine and methyl iodide is easy to leave under alkaline conditions. The heating reaction generates an intermediate, and the intermediate reacts with sodium selenomethoxide to obtain the final product L-selenomethionine, forming a more concise synthesis route of L-selenomethionine.
[0022] The synthetic route of the inventive method is: .
[0023] The method of the present invention comprises two steps of step A and step B.
[0024] Step A: Reaction of L-methionine and methyl iodide in water to obtain an intermediate, wherein the reaction temperature is 0-60° C., and the reaction time is 3-10 hours.
[0025] The molar ratio of L-methionine to methyl iodide is 1:1-1.5.
[0026] During the specific operation of this step, suspend L-methionine in water, add iodomethane dropwise at room temperature after dissolving,...
Embodiment 1
[0028] Suspend 298g of methionine (2mol) in 800ml of water, dissolve and add 340.8g (2mol) of methyl iodide dropwise at room temperature, heat to 40°C for 4 hours, a large amount of white solid precipitates, filter, and wash the filter cake with water to obtain 570.36g of white product , yield 98%, purity 99.3%.
[0029] Step B, the intermediate reacts with sodium selenomethoxide in an alcoholic solvent. After the reaction, glacial acetic acid is quenched to adjust the pH value of the reaction system to 5-6. After filtering and drying, L-selenomethionine is obtained. Among them, alcohol The quasi-solvent is methanol, ethanol or isopropanol, the reaction temperature is 0-50°C (preferably 30-50°C), and the reaction time is 4-10 hours.
[0030] The ratio of the intermediate to the sodium selenium methoxide in molar ratio is 1:0.8~4, preferably 1:0.9~2.
[0031] The specific operation process is as follows: under the protection of nitrogen, the intermediate is added to the alcoho...
Embodiment 2
[0035] Under the protection of nitrogen, the intermediate (16g, 55.2mmol) was added to absolute ethanol (350ml), and a methanol solution of sodium selenium methoxide (25.74g, 0.22mol) was added dropwise at 40-45°C. After the addition was completed, 40-45 °C for 6 hours. After the reaction was complete, lower the system to 20-25°C, add glacial acetic acid (15g) dropwise, adjust the pH to 5-6, filter, and dry to obtain 16.9g of white solid, yield 78%, HPLC purity 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com