Construction of CD3 specific lentivirus and application thereof
A recombinant virus, l1-z1-scfv-z2-tm-z3 technology, applied in the field of medical biology, can solve the problems of complicated and cumbersome process, difficult to popularize CAR-T cell therapy, and long preparation time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
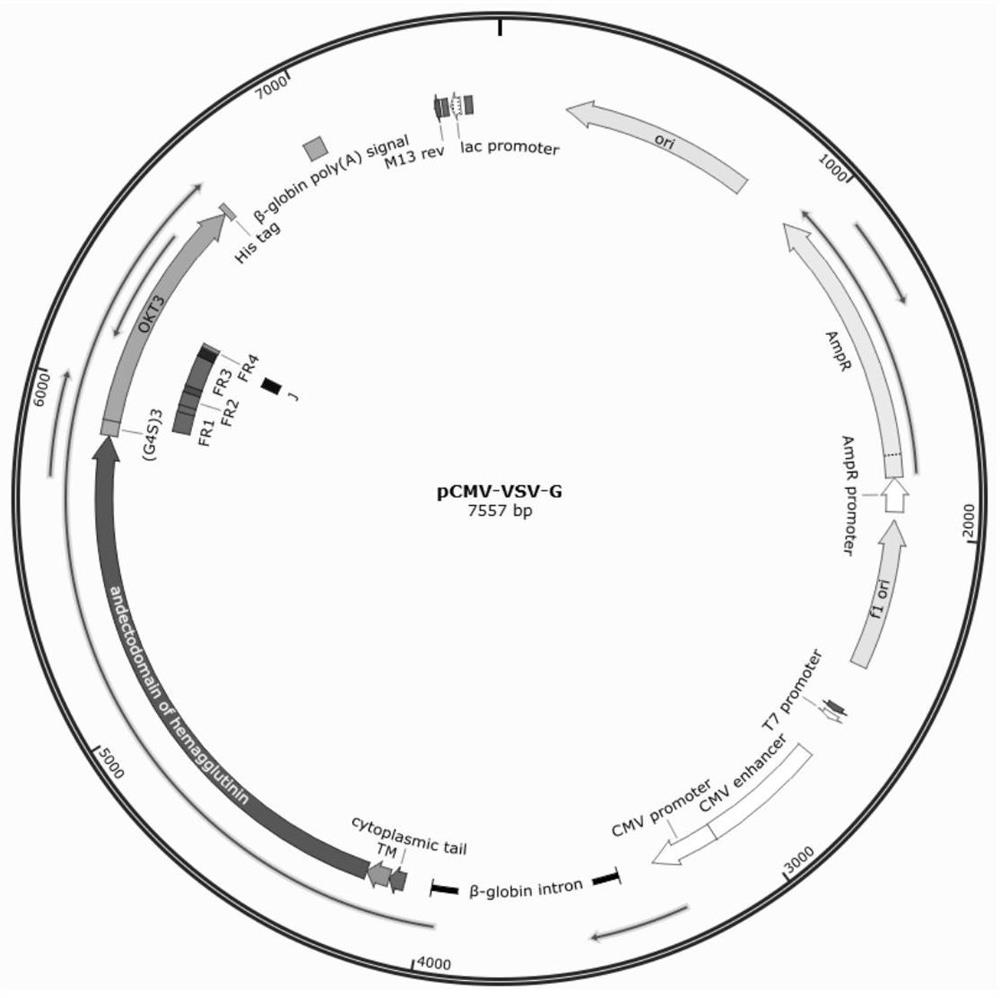
[0236]Example 1 CD3 specific transformation of a slow viral envelope protein (plasmid structurefigure 1 Indicated by)
[0237]1.1 Synthesis of sequence of hemorrhoids and associated primers
[0238]The nucleic acid sequence of leprosy virus hemagodile protein sends a living organism. Design-related primers are shown in Table 1, allowing it to add appropriate Linker between H protein and OKT3 during PCR.4S)3. Synthetic primer 4000rpm Centrifuge 5min to add the appropriate amount of DDH2O, the primer is finally concentrated to 10 um.
[0239]Table 1. Another primer, sequencing primers and identification primers for the construction of plasmids
[0240]
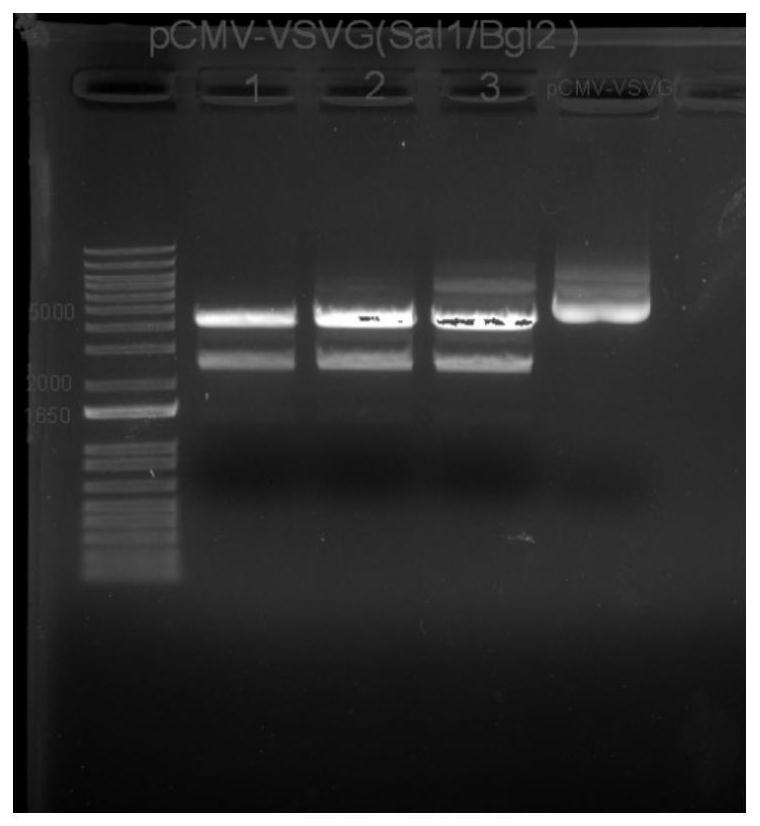
[0241]1.2 Double-CMV-VSVG (SalI, BglI)
[0242]After the plasmid P-CMV-VSVG is incubated with restriction endonuclease SALI, BGLI, the electrophoresis cream product, the electrophoresis of gel imaging resultsfigure 2 The colloidal recovery linear carrier P-CMV-VSVG (SALI, BGLII) product, size of about 4063 bp (base pair).
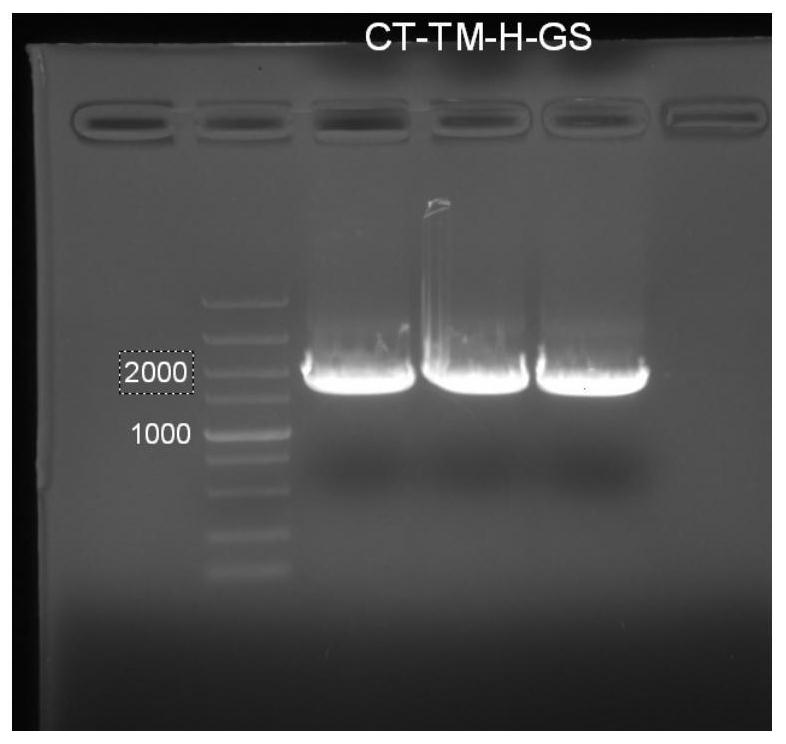
[0243]1.3 PCR amplification of ...
Embodiment 2
[0268]Example 2 Packaging Production Slow Virus
[0269]2.1 293FT cell culture
[0270]293FT cells are provided by Fujian Medical University Immunotherapy Research Institute, and the recovery in liquid nitrogen tanks. After washing to wash away the freeze-saving solution, add about 5 ml of DMEM complete medium, and take about 2 × 10 after counting.6Cell293FT cells in 75cm2About 10 ml of DMEM complete medium is added to the culture bottle, CO2Culture of constant temperature incubator.
[0271]2.2 Slow Virus PCDH-GFP-VSVG and PCDH-GFP-HMO Packaging Production
[0272]One day before transfection, 293FT cells in digestive state, 6-well plate 4-hole cells, according to 4 × 105Cell / hole average holes, 37 ° C CO2Culture of constant temperature incubator. The second day of the microscope views the cells, and the transfection can be carried out at between 70% -90%. DMEM was completely cultured with DMEM before transfection. Take 2 EP tubes, labeled VSVG groups (positive controls), and the amount of pl...
Embodiment 3
[0280]Example 3 Slow viral infection
[0281]3.1 Choose a suitable cell
[0282]293FT cells and Jurkat cells were selected for experiments, 293FT cells and Jurkat cells were provided by Fujian Medical University Immunology Institute. 293FT cells are human embryonic renal cells and does not express CD3. Jurkat cells are human peripheral blood hyzogeneous T cells, stably expressing CD3.
[0283]3.2 Slow virus infection 293FT / jurkat cells
[0284]Ten wells with a good state of 293FT and Jurkat cells, and count the 9 holes of the 24-well plates. Add a virus stock solution infection to the experimental group cells (see Table 5 for details).
[0285]Table 5. Each experimental group slow virus stock solution when infected with 293 ft / jurkat cells
[0286] cell VSVG group (UL) HMO group (UL) Blank control 293FT 7575Unable Jurkat7575Unable
[0287]The blank control group added isometric DMEM complete medium / 1640 fully medium, sealing film seal 24-well plate, 2000 rpm, 37 ° C centrifugation 90mins, re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com