Standard substance containing free-state and conjugated-state clenbuterol in swine urine and preparation method of standard substance
A standard substance, free state technology, used in material separation, measurement devices, instruments, etc., can solve the problems of inaccuracy, affect the accuracy, reliability and comparability of test results, and exceed standards, and achieve good application prospects and good social benefits. And economic benefits, good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Standard substance candidate preparation
[0031] In a pig farm with a scale of about 1000 pigs, 12 representative ordinary fattening pigs (4-5 months old, 2-4 weeks before slaughter, body weight about 120kg) were randomly selected as representative samples for parallel experiments. After formulating feed containing a certain concentration of clenbuterol, feed pigs regularly. At the same time, pig urine was continuously collected in clean sample bottles to determine the content of clenbuterol. Through the continuous and uninterrupted detection of pig urine samples obtained from 12 samples, it was found that the following general rules were found. After 3 hours of feeding, clenbuterol could be detected in pig urine; during 3-72 hours, The concentration of clenbuterol in pig urine was in a continuous rising period; the content was stable after 72h. After stopping feeding, it was in a rapid decline period within 3-48 hours; after 48 hours, the content of clenbu...
Embodiment 2
[0041] Embodiment 2 homogeneity test
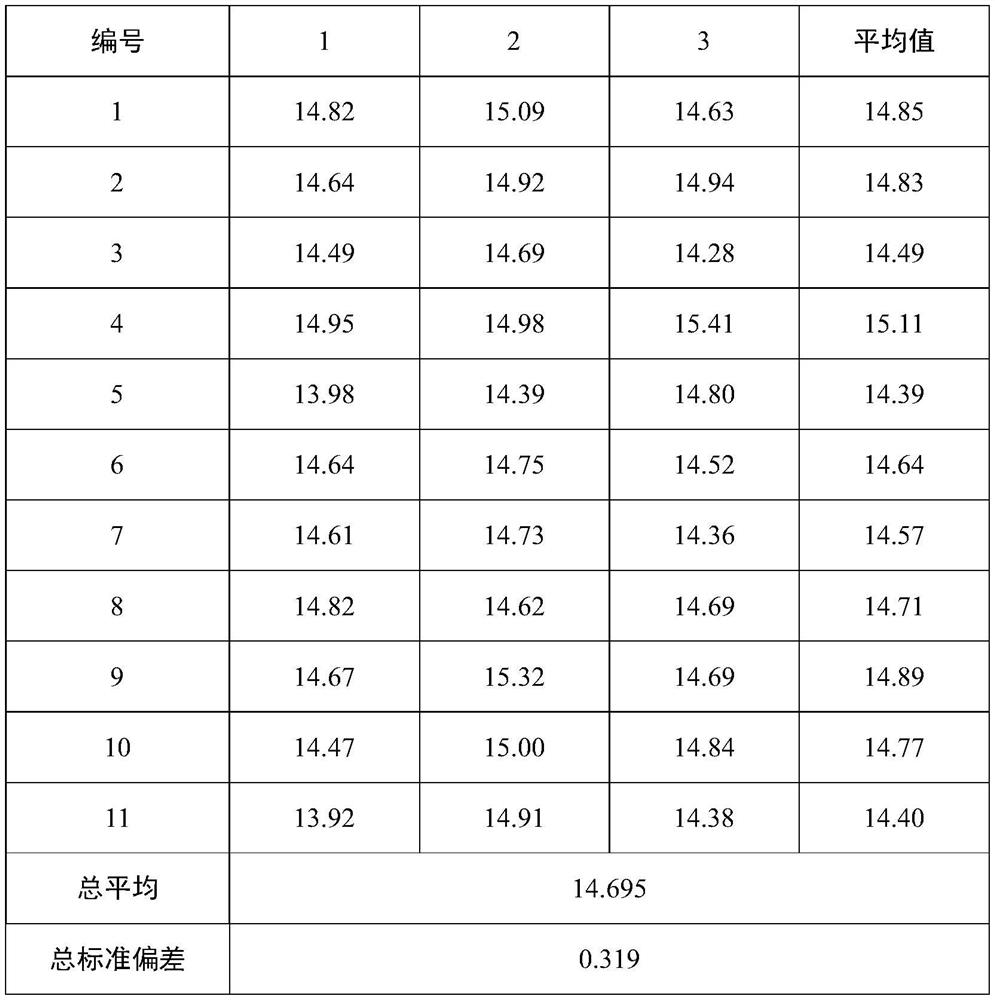
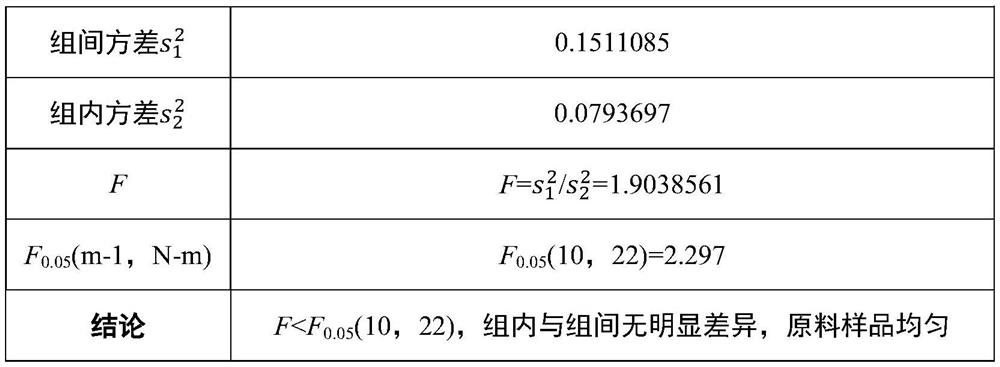
[0042] Randomly select 11 packaging units according to the whole packaging process before, during and after, and the randomly selected samples are numbered from 1 to 11, and each randomly selected unit takes 3 sub-samples in parallel, and the record numbers are 1-1, 1-2 , 1-3, 2-1, 2-2, 2-3, ..., 11-1, 11-2, 11-3. The homogeneity test method adopts liquid chromatography-isotope dilution mass spectrometry to measure the results (see the standard substance determination part for specific method parameters), and the results are statistically analyzed by analysis of variance, and judged by comparing the F test value with the F critical value. See Table 1 for the results of the homogeneity test and statistical analysis of the clenbuterol components in pig urine.
[0043] Table 1 Pig urine matrix standard substance homogeneity test results containing clenbuterol (ng / mL)
[0044]
[0045]
[0046] The above experimental data show that th...
Embodiment 3
[0047] Embodiment 3 Stability
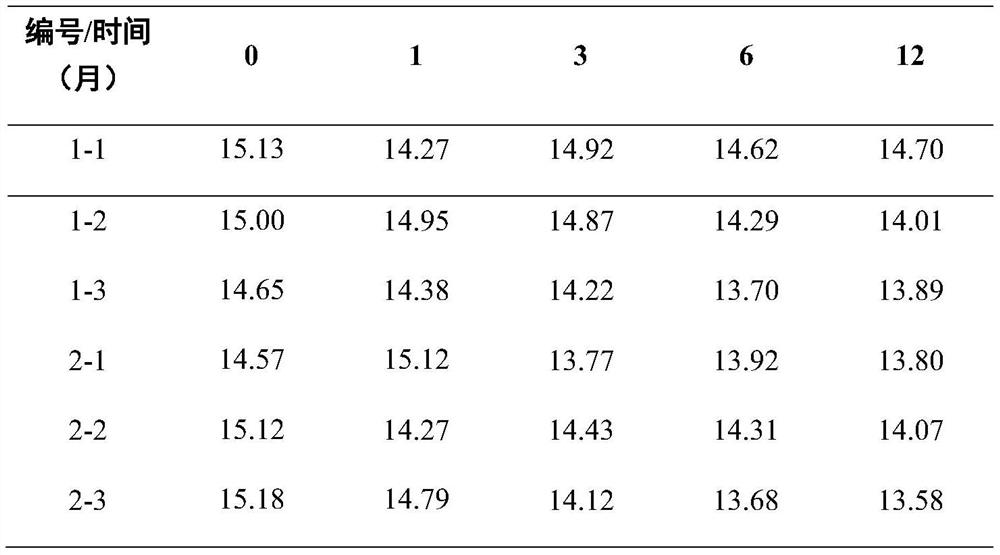
[0048] Long-term stability monitoring studies were carried out at 0, 1, 3, 6, 9, and 12 months, respectively. Three packaging units are randomly selected each time, and each unit is measured in parallel three times. The measurement method is the same as that used in the homogeneity test, both of which are liquid chromatography-isotope dilution chromatography mass spectrometry. The average value of the measurement results of the three packaging units is taken as the long-term stability monitoring result. The trend analysis method is used to analyze the results, and the monitoring time and the results are fitted with a straight line, and the results are statistically analyzed. The long-term stability monitoring results of the pig urine freeze-dried powder containing clenbuterol are shown in Table 2. A straight line was fitted with the detection time and results, and the trend analysis method was used to statistically analyze the stability test res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com