Chronic wound healing composition and application thereof
A technology for chronic wounds and pharmaceutical compositions, applied in the direction of drug combinations, active ingredients of hydroxyl compounds, active ingredients of heavy metal compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0055] Drugs: (1) Acteoside (Acteoside, code-named A): purchased from Sigma-Aldrich (St.Louis, MO, USA).
[0056] (2) Bismuth subgallate (Bismuth subgallate, code-named BSG): American Sigma-Aldrich (St.Louis, MO, USA).
[0057] (3) Borneal (code-named BO): American Sigma-Aldrich (St.Louis, MO, USA).
[0058] According to the present invention, compositions can be prepared according to the following composition:
[0059]
[0060]
[0061] Carry out the content of each component of the composition of the present invention of following cell experiment:
[0062] (1) Acteoside 16.0 μM (0.01 μg / μl), that is, 0.1%;
[0063] (2) Bismuth subgallate 14.2 μM (0.0056 μg / μl), ie 0.056%; and
[0064] (3) Borneo BO 18.8 μM (0.0029 μg / μl), ie 0.029%.
[0065] Cell culture:
[0066] Human dermal fibroblast (HDF cells) or skin keratinocytes (human keratinocytes, HaCaT cells) were cultured in a humid incubator at 37°C in an atmosphere of 5% carbon dioxide and 95% oxygen. Place in Dul...
example 1
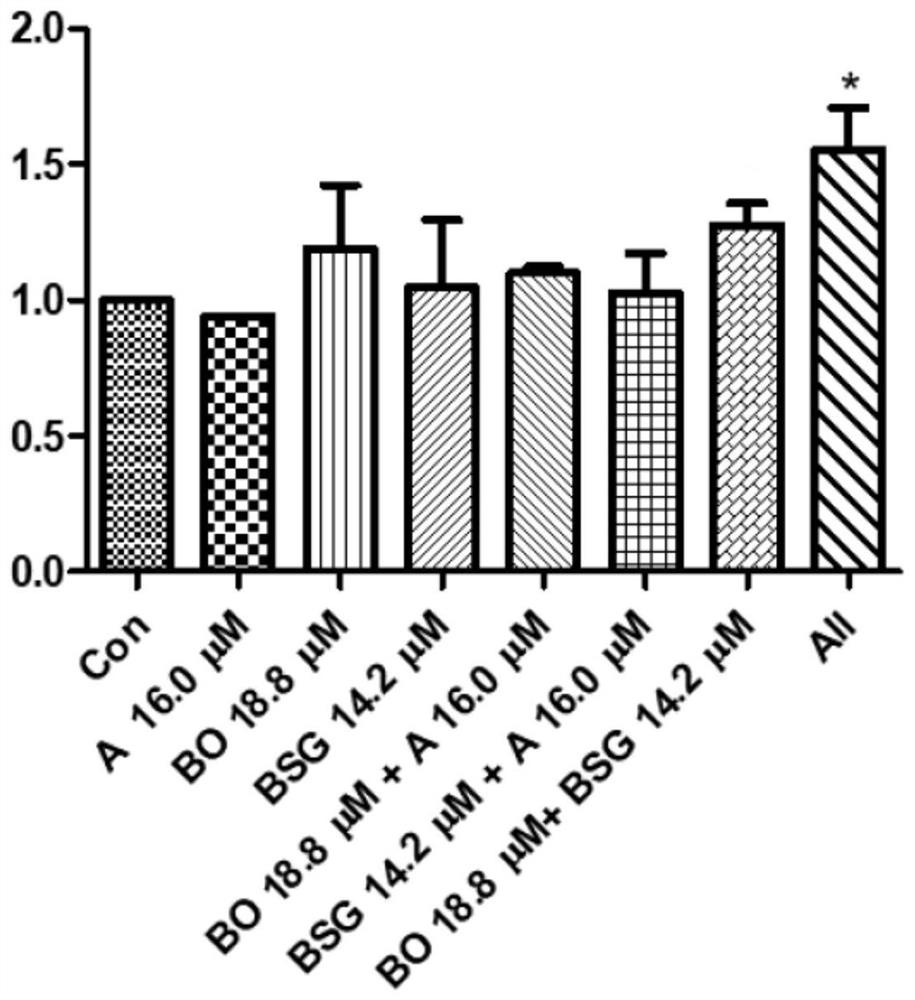
[0067] Example 1 Cell Growth Analysis (Cell Proliferation Assay)
[0068] Take HaCaT cells 3 x 10 5 Add 25mM glucose to the standard and culture in a 12-well petri dish for 48 hours. Cell growth and viability were analyzed with the CCK-8 reagent kit (Dojindo Molecular Technologies, Kumamoto, Japan), 5 μL of CCK-8 solution was added to each cell and incubated at 37°C for one hour, and then the spectrophotometer Microplate reader (SpectraMax, Molecular Device, USA) was used to measure the absorption at 450nm, and the sample was compared with the value of the control group to analyze the experimental results. The experimental groups tested included:
[0069] (1) Con: control group;
[0070] (2) A 16.0 μM;
[0071] (3) BO 18.8μM;
[0072] (4) BSG 14.2μM;
[0073] (5) BO 18.8μM+A 16.0μM;
[0074] (6) BSG 14.2μM+A 16.0μM;
[0075] (7) BO 18.8μM+BSG 14.2μM;
[0076] (8) All (A 16.0 μM+BO 18.8 μM+BSG 14.2 μM).
[0077] The results of cell proliferation after 48 hours of cult...
example 2
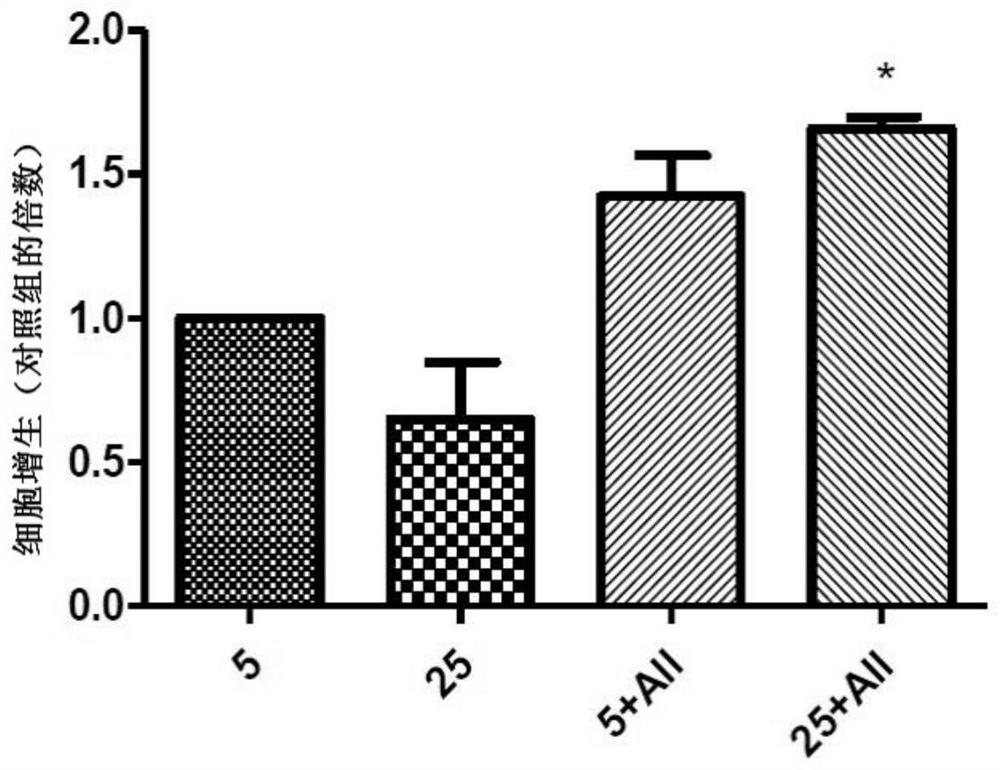
[0078] Example 2 low sugar and high sugar environment cell culture analysis comparison (Comparison in Cell Proliferation Assay)
[0079] Take HaCaT cells 3 x 10 5 Cultured with 5mM and 25mM glucose, cultured in a 12-well petri dish for 48 hours, added the composition of the present invention (All:A 16.0μM+BO 18.8μM+BSG 14.2μM) to observe the cell proliferation status.
[0080] The results of cell proliferation after 48 hours of culture are as follows: figure 2 , showing that the composition of the present invention (A 16.0μM+BO 18.8μM+BSG 14.2μM) has a significant synergistic effect on cell growth in high-sugar culture (25mM), but does not significantly promote cell proliferation in low-sugar (5mM) effect.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com