Preparation method of (R)-3-(3-chloro-2-fluorobenzene)-4, 5-dihydroisoxazole-5-carboxylic acid
A technology of dihydroisoxazole and isoxazole is applied in the directions of organic chemical methods, chemical instruments and methods, organic racemization, etc., and achieves the effects of high reaction yield and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
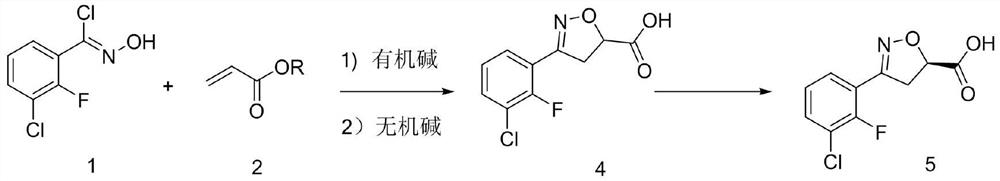
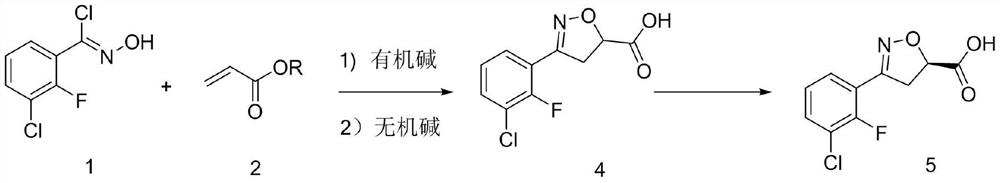
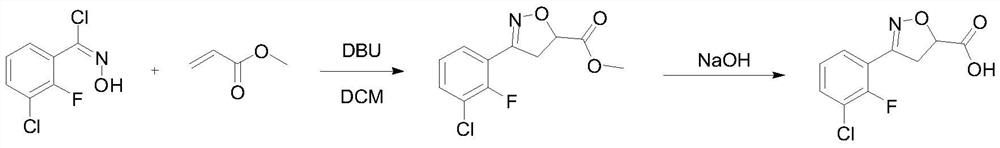
[0026] Under nitrogen protection, 104 g (0.5 mol) of 3-chloro-2-fluoro-o-chlorobenzaldoxime and 500 mL of dichloromethane were put into the reaction flask. The material was dissolved under stirring at room temperature, cooled to 10°C, 77.5 g (0.9 mol) of methyl acrylate was added, and 83.7 g (0.55 mol) of DBU was added dropwise. After the addition was completed, the temperature was slowly raised to room temperature for 8 hours, and the raw material was detected by HPLC at 0.9% .
[0027] Add saturated ammonium chloride aqueous solution to quench, separate layers, extract the aqueous phase once with dichloromethane, combine the organic phases, wash the organic phase with 0.5N hydrochloric acid and saturated brine, concentrate the organic phase until it is stagnant, add 300 mL of absolute ethanol . The material was dissolved under stirring at room temperature, cooled to 5°C, and 134.6 g (0.673 mol) of 20% sodium hydroxide aqueous solution was slowly added dropwise, ...
Embodiment 2
[0029]
[0030] Under nitrogen protection, 104 g (0.5 mol) of 3-chloro-2-fluoro-o-chlorobenzaldoxime and 500 mL of 2-methyltetrahydrofuran were put into the reaction flask. The material was dissolved under stirring at room temperature, cooled to 10°C, 102.7 g (0.9 mol) of isopropyl acrylate was added, 83.7 g (0.55 mol) of DBU was added dropwise, and after the addition was completed, the temperature was slowly raised to room temperature for 8 hours of reaction. %.
[0031] Add saturated ammonium chloride aqueous solution to quench, separate layers, extract the aqueous phase once with 2-methyltetrahydrofuran, combine the organic phases, wash the organic phase with 0.5N hydrochloric acid and saturated brine, cool the organic phase to 5 °C, slowly add 20 % sodium hydroxide aqueous solution 134.6g (0.673mol), after the dropwise addition was completed, the temperature was slowly raised to room temperature for reaction for 4 hours, and 0.2% of the raw material was detected by HPLC...
Embodiment 3
[0033]
[0034] Under nitrogen protection, 104 g (0.5 mol) of 3-chloro-2-fluoro-o-chlorobenzaldoxime and 500 mL of diethoxymethane were put into the reaction flask. The material was dissolved under stirring at room temperature, cooled to 10°C, 90 g (0.9 mol) of ethyl acrylate was added, and 83.7 g (0.55 mol) of DBU was added dropwise. After the addition was completed, the temperature was slowly raised to room temperature for 8 hours of reaction, and 0.2% of the raw material was detected by HPLC.
[0035] Add saturated ammonium chloride aqueous solution to quench, separate layers, extract the aqueous phase once with diethoxymethane, combine the organic phases, wash the organic phase with 0.5N hydrochloric acid and saturated brine, cool the organic phase to 5 ° C, slowly add 20 % sodium hydroxide aqueous solution 188.8g (0.673mol), after the dropwise addition was completed, the temperature was slowly raised to room temperature for reaction for 4 hours, and 0.2% of the raw mate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com