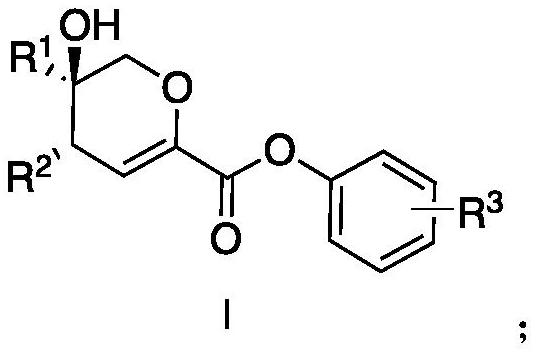
Chiral dihydropyran ring derivative as well as preparation method and application thereof
A technology of dihydropyran rings and derivatives, applied in the direction of drug combinations, pharmaceutical formulations, organic active ingredients, etc., to achieve good inhibitory effect, excellent inhibitory effect, and good anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Preparation of Chiral Dihydropyran Ring Derivatives
[0052] The specific preparation reaction is as follows:
[0053]
[0054] The hydroxy ketone (0.30 mmol) represented by formula 2, the chiral metal rhodium (0.009 mmol, metal catalyst), Molecular sieves (300mg) were dissolved in 3.0mL of organic solvent dichloromethane to configure mixed solution 1, and the diazo compound (0.6mmol) shown in formula 1 was dissolved in 3.0mL of organic solvent dichloromethane to configure solution 2; in- At 60°C, add solution 2 to the aforementioned mixed solution 1 with a syringe pump within 1 hour, and stir vigorously; after the dropwise addition of the mixed solution is completed, stir at -60°C for 5 to 8 hours until the diazo compound is completely consumed; Filtration, column chromatography separation and purification, to obtain the pure product, namely the target product.
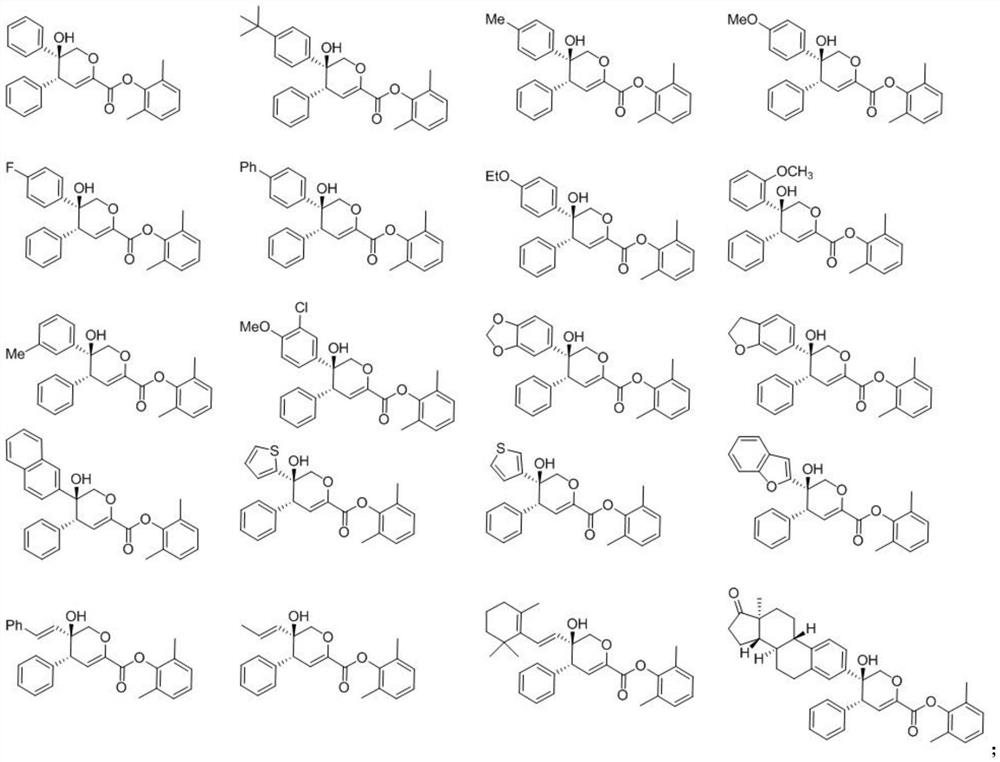
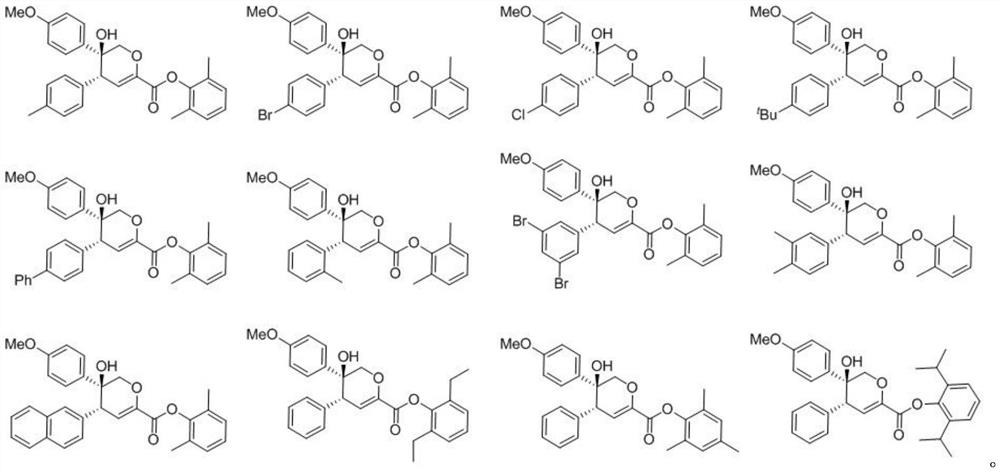
[0055] The structures of the prepared compounds 1 to 32 are shown in Table 1, and the speci...
Embodiment 2
[0092] Example 2 Inhibitory activity of chiral dihydropyran ring derivatives on tumor cells
[0093] (1) The tumor cells used in the test are: human osteosarcoma cells (Sjsa-1), human colon cancer cells (HCT116), human non-small cell lung cancer cells (A549), and human osteosarcoma cells (Saos-2).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com