Construction method of porcine TFF1 gene knockout cell line based on CRISPR-Cas9 gene editing technology
A cell line and gene technology, applied in the field of genetic engineering, can solve the problem that there is no pig small intestinal epithelial cell model
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
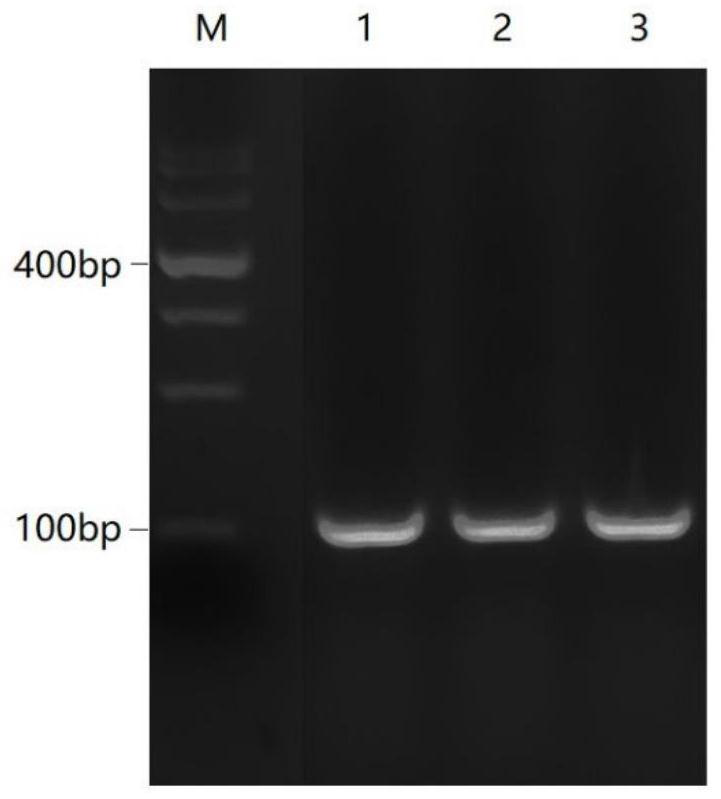
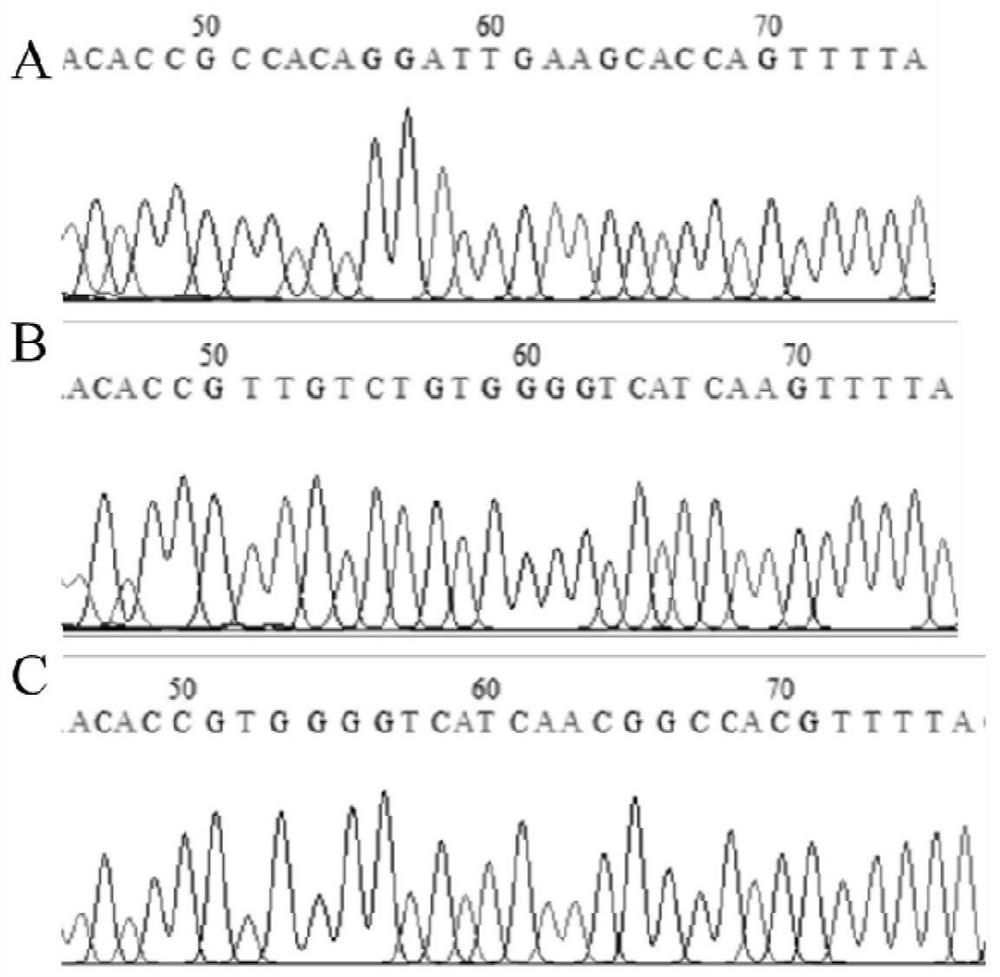
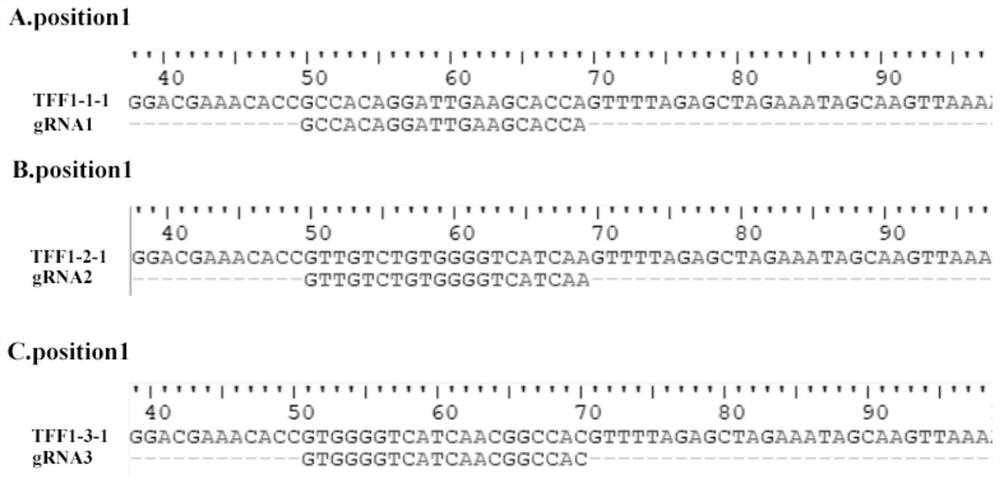
[0049] 1. Target site design and sgRNA sequence synthesis:
[0050] According to the NCBI database (https: / / www.ncbi.nlm.nih.gov / ), the transcript CDS sequence of the porcine TFF1 gene (accession number: XM_003358973.3) was obtained, and the knockout target site was designed according to the 5' end of the CDS region , using CRISPRDesign (http: / / crispr.mit.edu / ) to design three sgRNA guide sequences sgRNA1, sgRNA2 and sgRNA3, sgRNA1: GCCACAGGATTGAAGCACCA;
[0051] sgRNA2: GTTGTCTGTGGGGTCATCAA;
[0052] sgRNA3: GTGGGGTCATCAACGGCCAC. The base CACC was added to the 5' end of the three sgRNA guide sequences to form a positive-strand sgRNA sequence; the three designed sgRNA guide sequences were reverse-complemented, and the base AAAC was added to the 5'-end to form a negative-strand sgRNA sequence. The three pairs of sgRNA sequences are: TFF1-1F: CACCGCCACAGGATTGAAGCACCA
[0053] TFF1-1R: AAACTGGTGCTTCAATCCTGTGGC
[0054] TFF1-2F: CACCGTTGTCTGTGGGGTCATCAA
[0055] TFF1-2R: AAAC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com