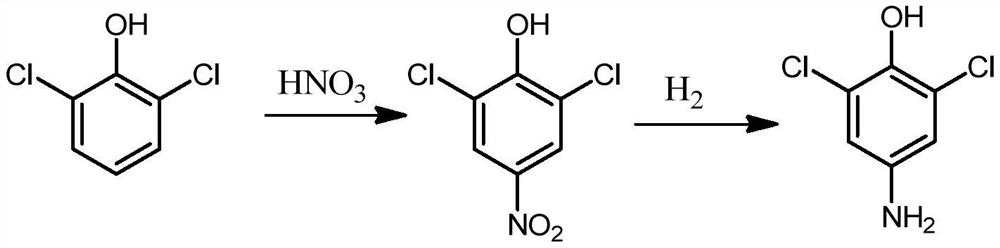
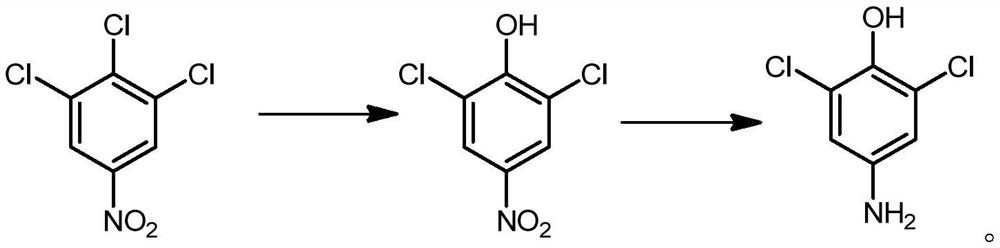
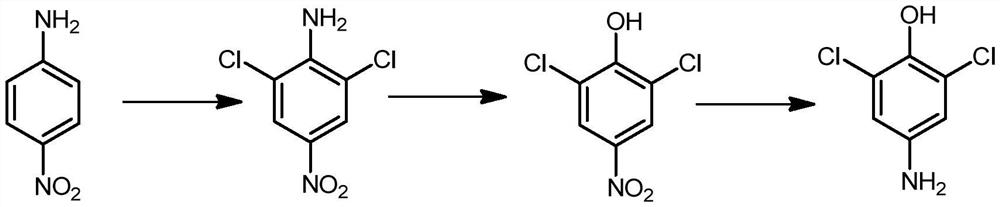
Synthesis method of 2, 6-dichloro-4-aminophenol
A technology of aminophenol and synthesis method, which is applied to the preparation of amino compounds, preparation of amino hydroxyl compounds, chemical instruments and methods, etc., can solve the problems of difficult hydrolysis and low hydrolysis selectivity, so as to prevent dechlorination and reduce distillation Risk and energy consumption, the effect of simplifying the process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Chlorination: Weigh 27.6g of p-nitroaniline and 138g of methanol into the flask, stir to dissolve, raise the temperature to 55°C, start slowly introducing chlorine gas, control the temperature at 55-60°C, gradually precipitate yellow solid, and monitor the raw materials by HPLC After the basic reaction, cool down to room temperature, filter with suction, wash the solid with water first, then wash with 2% soda ash solution until neutral, filter with suction, and dry to obtain 40g of 2,6-dichloro-4-nitroaniline, the appearance is Bright yellow solid, HPLC purity 98.4%, molar yield 96.62%.
[0033] (2) Diazo hydrolysis: Weigh 150g of 98% sulfuric acid and 158.7g of nitrososulfuric acid solution (42%) into the flask, stir, cool to 5°C, slowly add 2,6-dichloro-4-nitro A solution composed of 103.5g of aniline and 310.5g of toluene, the reaction temperature is controlled at 10-15°C, after the addition is completed, the reaction is kept at 10-15°C for 1 hour to obtain a cle...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com