Hepatitis B immunotherapy agent targeting ccl19/ccr7 and its use
A-CCL19, hepatitis B technology, applied in the field of biomedicine, can solve problems such as the role has not yet been announced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
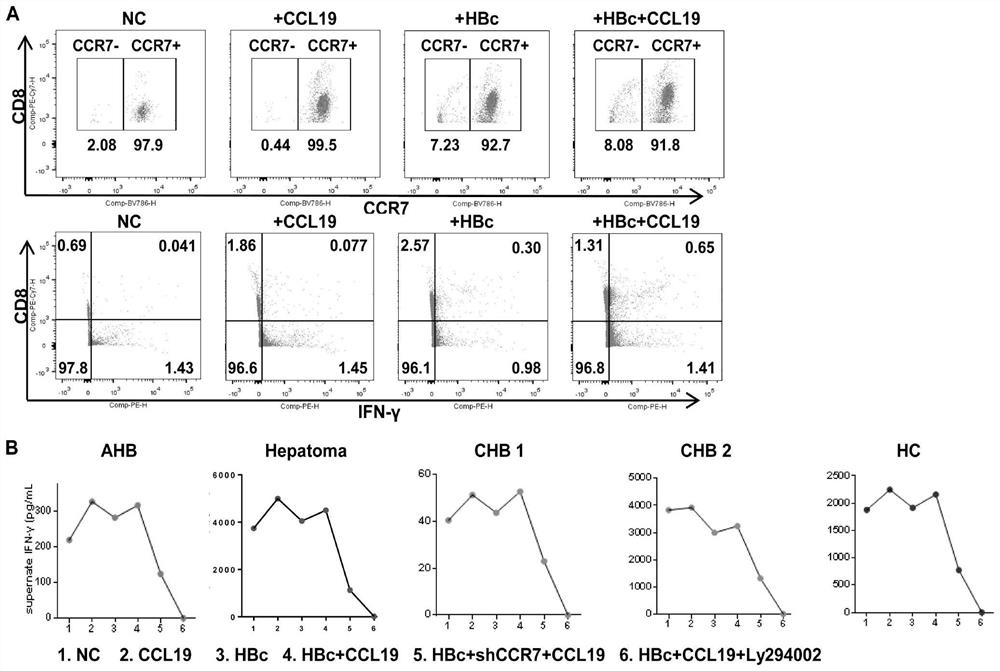
[0048] Example 1 Immunomodulators CCL19 and shCCR7 are used for peripheral blood lymphocytes of patients with HBV chronic infection
[0049] The reason why the virus in the liver of patients with chronic hepatitis B cannot be cleared is mainly because the liver is an immune tolerance organ, and at the same time, there is exhaustion of CD8+ T lymphocytes in patients with hepatitis B. Functional validation was performed on peripheral blood lymphocytes (PBMC). The specific steps are:
[0050] (1) Extract peripheral blood lymphocytes and add them to 24-well plate, 1×10 per well 6 Lymphocytes per 500ml, CCL19 group was stimulated with human CCL19 protein (10ng / well) for 72 hours, HBc group was stimulated with HBV specific antigen HBc polypeptide (10ng / well) for 72h; HBc+CCL19 group was stimulated with human CCL19 protein (10ng / well) and HBV-specific antigen HBc polypeptide stimulation for 72h; HBc+CCL19+L294002 group was stimulated with human CCL19 protein (10ng / well) and HBV-spe...
Embodiment 2
[0051] The establishment of embodiment 2 mouse model
[0052] C57 / BJ mice chronically infected with HBV were used to construct the HBV-infected mouse model, and the HBV1.2 plasmid was injected through the high-pressure tail vein, 10ng / mouse. LinYJ, Huang LR, Yang HC, Tzeng HT, Hsu PN, Wu HL, et al. Hepatitis B virus coreantigen determines viral persistence in a C57BL / 6mouse model. Proceedings of the National Academy of Sciences of the United States of America (2010) 107 (20):9340-5. doi:10.1073 / pnas.1004762107.
[0053] The HBV-infected mouse model constructed according to the above method was used to construct a model with high expression of CCL19 and decreased expression of its receptor CCR7.
[0054] (1) Mouse model of chronic HBV infection with high expression of CCL19 molecules: the recombinant expression mouse CCL19 plasmid on pcDNA3.1(+) constructed in our laboratory (plasmid map as shown in Figure 7所示,编码CCL19的核苷酸序列为:ATGGCCCCCCGTGTGACCCCACTCCTGGCCTTCAGCCTGCTGGTTCTCTG...
Embodiment 3
[0057] Example 3 Immunomodulators CCL19 and shCCR7 are used for HBV chronically infected mice
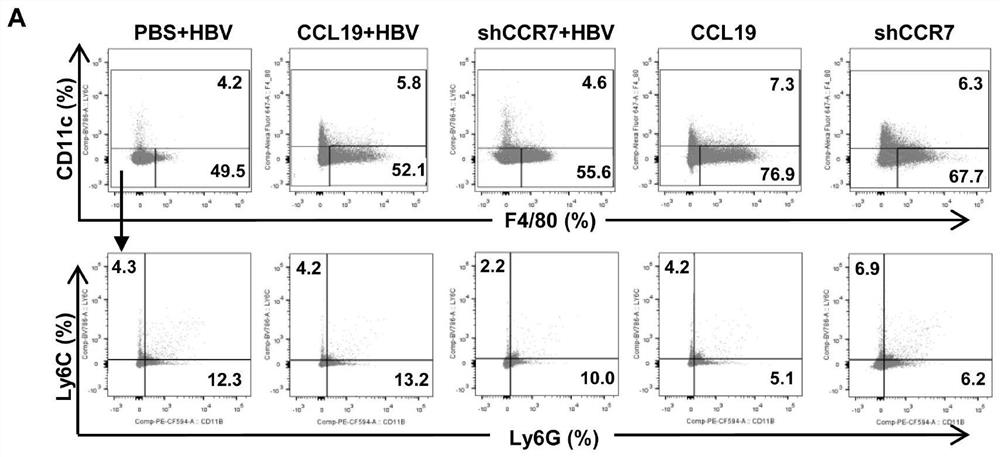
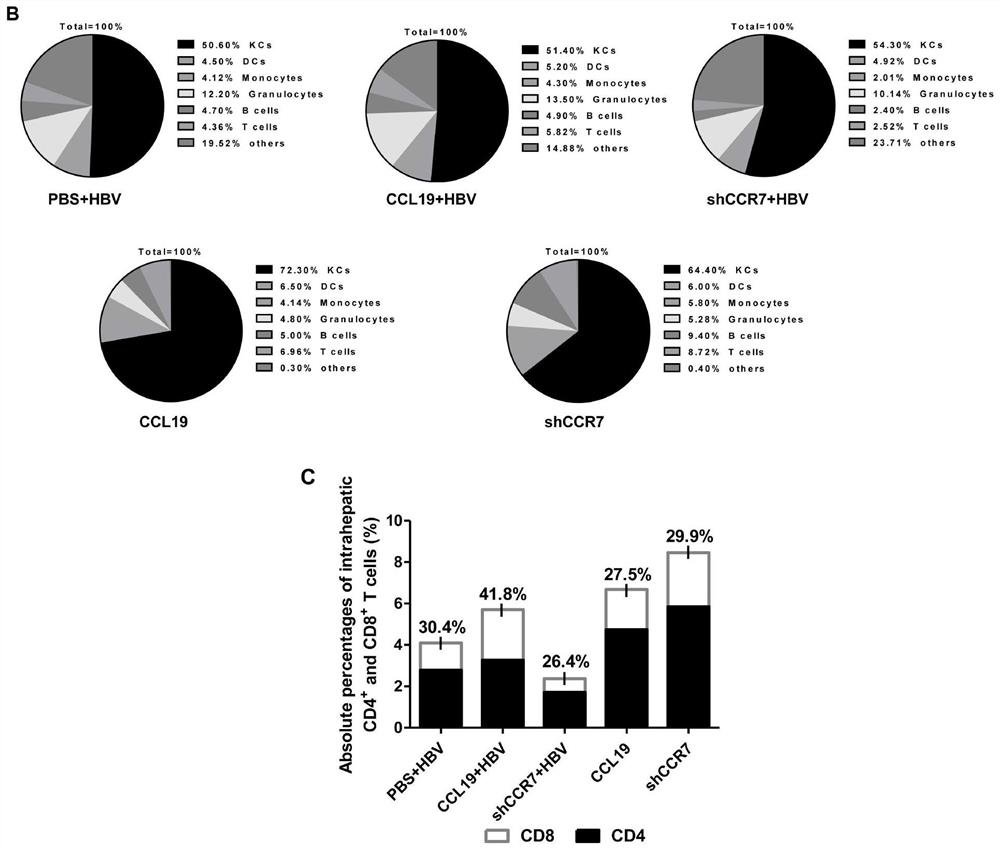
[0058] The immunomodulator CCL19 and the immunomodulator shCCR7 were functionally verified on HBV chronically infected mice, and the control mouse model constructed in Example 2, the CCR7 knockdown HBV chronically infected mouse model and the high expression of CCL19 molecules were detected respectively The proportion of liver non-parenchymal cells, immune modulation and the effect on cells in chronic HBV infection mouse model.
[0059] (1) Changes in the proportion of liver non-parenchymal cells 2 weeks after the action of immunomodulators: the proportion of KCs cells (F4 / 80+, CD11c-) in mice with high expression of CCL19 was significantly increased (about 70%), and the molecular knockdown of CCR7 Cause the proportion of KCs cells to decline (about 60%), and the proportion difference in each group of CHB mice is not obvious (about 50%) ( figure 2 A-2B). However, compared with th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com