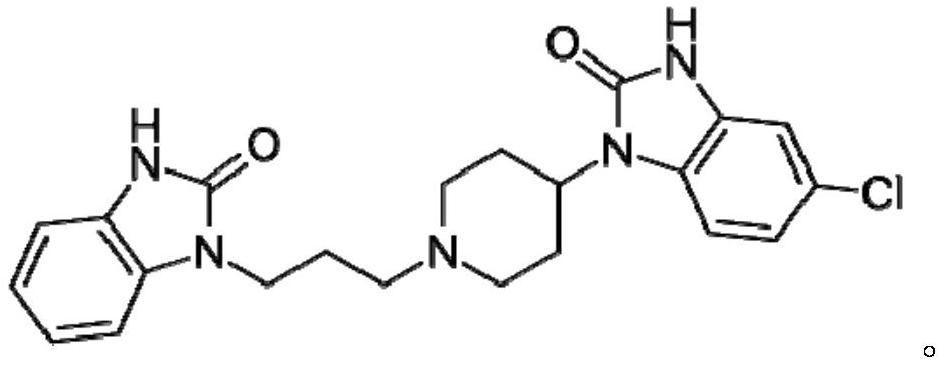
Formulations containing domperidone
A technology for domperidone and preparations, applied in the field of preparations containing domperidone, can solve the problems of limited accessibility of cisapride, easy loss of efficacy of erythromycin, adverse interaction and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
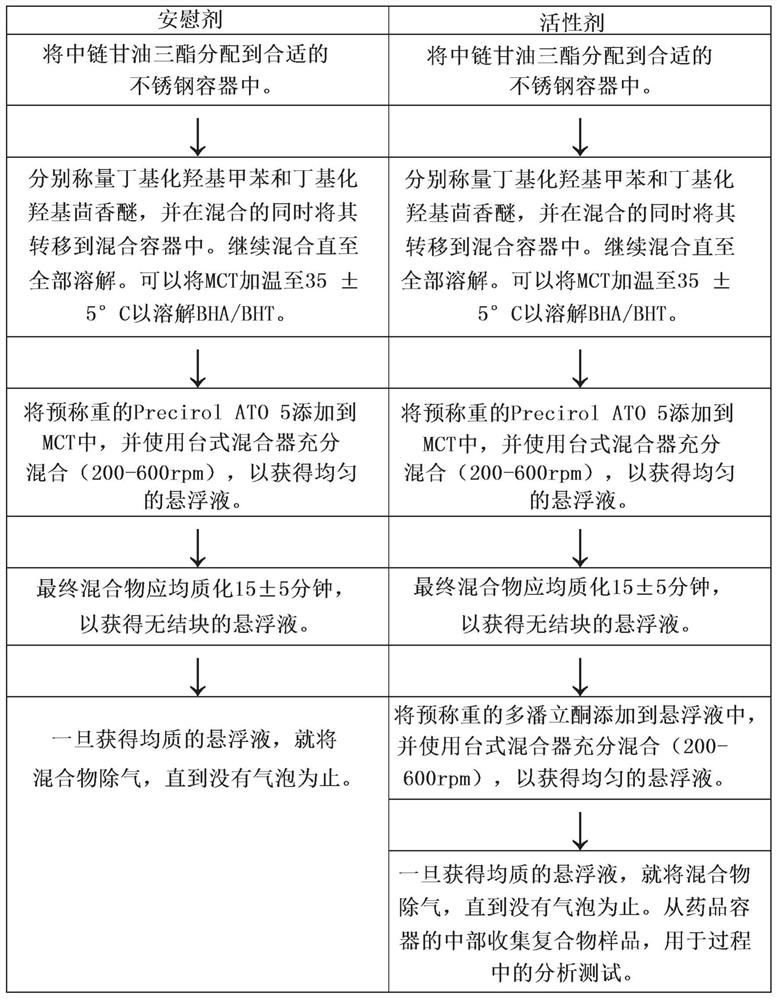
[0066] Embodiment 1: formulation preparation
[0067] By combining the components indicated in Table 1, five formulations containing domperidone, A-E, were prepared.
[0068]
[0069] Formulations A-C and E were formulated as liquids or semi-solids and filled into capsules, Formulation D was compressed using a single station carver press to form tablets.
Embodiment 2
[0071] Domperidone pharmaceutical formulations were prepared and encapsulated on a GMP encapsulation machine using the quantities noted in Tables 2-4. Specifically, batching activities were performed under a nitrogen blanket and yellow light. The batch was then encapsulated using a 2C oval mold and a 0.040" hole single bottom shot wedge at a temperature of about 38.7 to 51.7°C. The capsules were filled with a medium chain triglyceride / lecithin mixture ( 97% MCT / 3% lecithin) hand polished.
[0072]
[0073]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com