Drug-loaded emulsion of liquid crystal coated crystal drug and preparation method thereof
A liquid crystal emulsification and drug technology, applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of poor biofilm permeability, poor smearing skin feel, low transdermal absorption rate, etc., to achieve high skin safety, Overcome permeability, overcome the effect of low transdermal absorption rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] A drug-loaded layered liquid crystal emulsion, comprising the following raw materials in parts by weight:
[0082] Lecithin liquid crystal emulsifier 1.0 parts
[0083] 10.0 parts of saturated isopropyl myristate
[0084] Ketoprofen drug 0.4 parts
[0085] Capo U21 0.2 parts
[0086] 0.4 parts of 10% sodium hydroxide
[0087] 0.5 parts of 1,2-hexanediol
[0088] Purified water 87.5 parts
[0089] The implementation steps of above-mentioned formula are:
[0090] (1) Weighing the following raw materials in parts by weight, i.e. the oil phase, and preheating it in a constant temperature water bath at 75-80°C;
[0091] Lecithin liquid crystal emulsifier 1.0 parts
[0092] 10.0 parts of saturated isopropyl myristate
[0093] Ketoprofen drug 0.4 parts
[0094] (2) Weigh the following raw materials in parts by weight, i.e. the water phase, and preheat it in a constant temperature water bath at 75-80°C;
[0095] Capo U21 0.2 parts
[0096] Add water to 100.0 parts
...
Embodiment 2
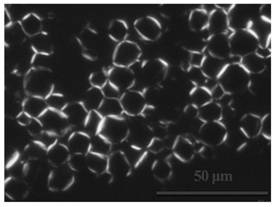
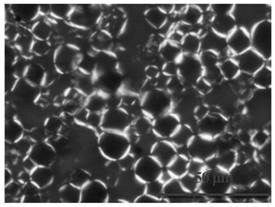
[0101] Operate the same as in Example 1, only changing the grease to isopropyl palmitate to prepare the drug-loaded layered liquid crystal emulsion, and the polarized light test results are as follows: figure 2 shown.
Embodiment 3
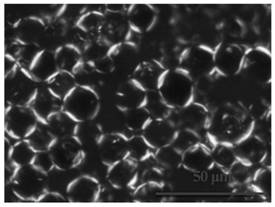
[0103] Operate the same as in Example 1, only change the oil into caprylic acid / capric triglyceride, and prepare the drug-loaded layered liquid crystal emulsion. The polarized light test results are as follows: image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com