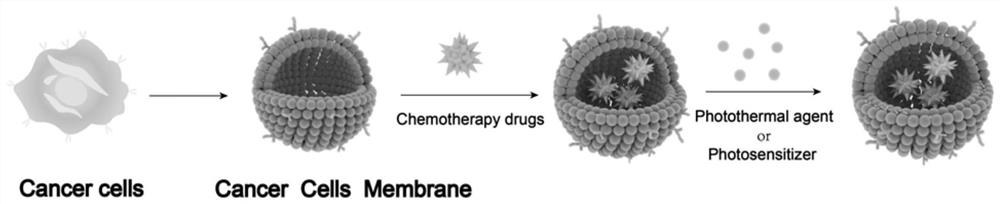
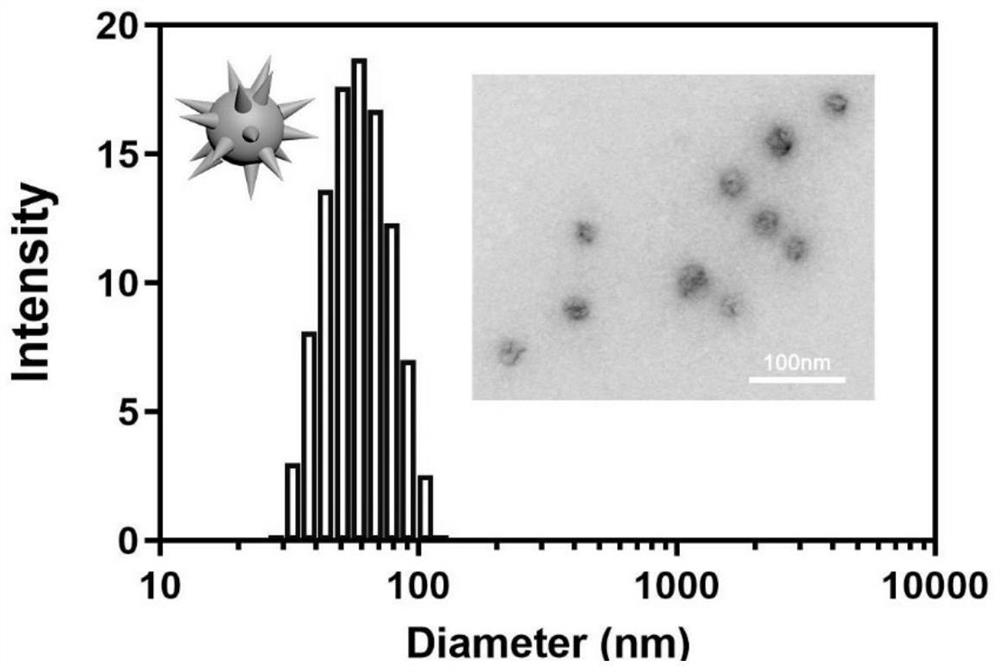
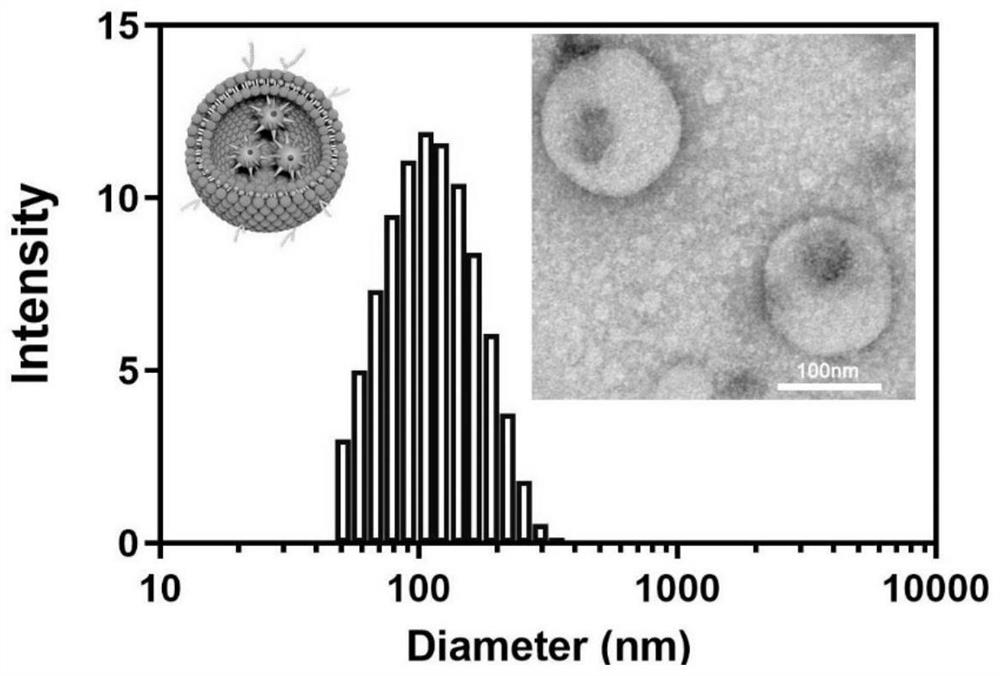
Bionic cell membrane nanoparticle as well as preparation method and application thereof
A cell membrane and nanoparticle technology, applied in the field of pharmaceutical preparations, can solve the problems of "cold" tumors, tumors evading immune system monitoring, etc., and achieve the effects of high production cost performance, powerful anti-tumor, and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of biomimetic cell membrane nanoparticles (CMM-DiR):
[0044] (1) Accurately weigh 4.6mg Na 2 S 2 o 3 Dissolved in 5mL water, 3.9mg KMnO 4 Dissolve in 5mL of water.
[0045] (2) Na in step (1) 2 S 2 o 3 Aqueous solution was added to KMnO 4 In the aqueous solution, the mixed solution was placed in a 50mL double-necked flask, nitrogen gas was introduced, and stirred on a magnetic stirrer for 45 minutes to initially obtain black MnO 2 precipitation.
[0046] (3) Rinse the black precipitate obtained in step (2) with purified water at 60°C, and then dry at 110°C for 1.5 hours to obtain MnO 2 nanoparticles.
[0047] (4) B16-F10 mouse melanoma cells were cultured in DMEM medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. When the confluence of the cells reached 80-90%, the cells were collected into the PBS solution by scraping, centrifuged at 700g for 7 minutes, and then resuspended in a 50:50 solution of cryopreservation med...
Embodiment 2
[0058] Preparation of biomimetic cell membrane nanoparticles (CMM-DiR):
[0059] (1) Accurately weigh 9mg Na 2 S 2 o 3 Dissolved in 10mL water, 7.5mg KMnO 4 Dissolve in 10mL of water.
[0060] (2) Na in step (1) 2 S 2 o 3 Aqueous solution was added to KMnO 4 In the aqueous solution, the mixed solution was placed in a 50mL double-necked flask, nitrogen gas was introduced, and stirred on a magnetic stirrer for 45 minutes to initially obtain black MnO 2 precipitation.
[0061] (3) Rinse the black precipitate obtained in step (2) with purified water at 60°C, and then dry at 110°C for 2 hours to obtain MnO 2 nanoparticles.
[0062] (4) B16-F10 mouse melanoma cells were cultured in DMEM medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. When the confluence of the cells reached 80-90%, the cells were collected into the PBS solution by scraping, centrifuged at 700g for 7 minutes, and then resuspended in a 50:50 solution of cryopreservation mediu...
Embodiment 3
[0068] Preparation of biomimetic cell membrane nanoparticles (CMM-DiR):
[0069] (1) Accurately weigh 5.2mg Na 2 S 2 o 3 Dissolved in 5mL water, 4.8mg KMnO 4 Dissolve in 5mL of water.
[0070] (2) Na in step (1) 2 S 2 o 3 Aqueous solution was added to KMnO 4 In the aqueous solution, the mixed solution was placed in a 50mL double-necked flask, nitrogen gas was introduced, and stirred on a magnetic stirrer for 60 minutes to initially obtain black MnO 2 precipitation.
[0071] (3) Rinse the black precipitate obtained in step (2) with purified water at 60°C, and then dry at 110°C for 2 hours to obtain MnO 2 nanoparticles.
[0072] (4) B16-F10 mouse melanoma cells were cultured in DMEM medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. When the confluence of the cells reached 80-90%, the cells were collected into the PBS solution by scraping, centrifuged at 700g for 7 minutes, and then resuspended in a 50:50 solution of cryopreservation medium ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com