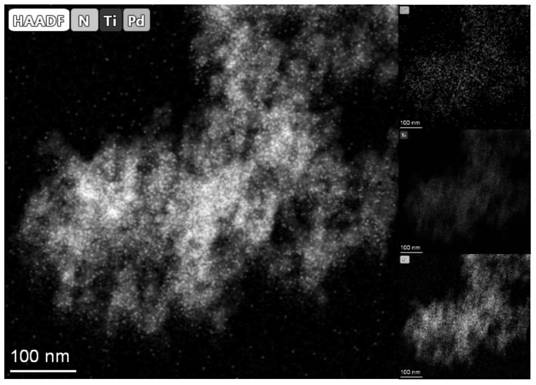
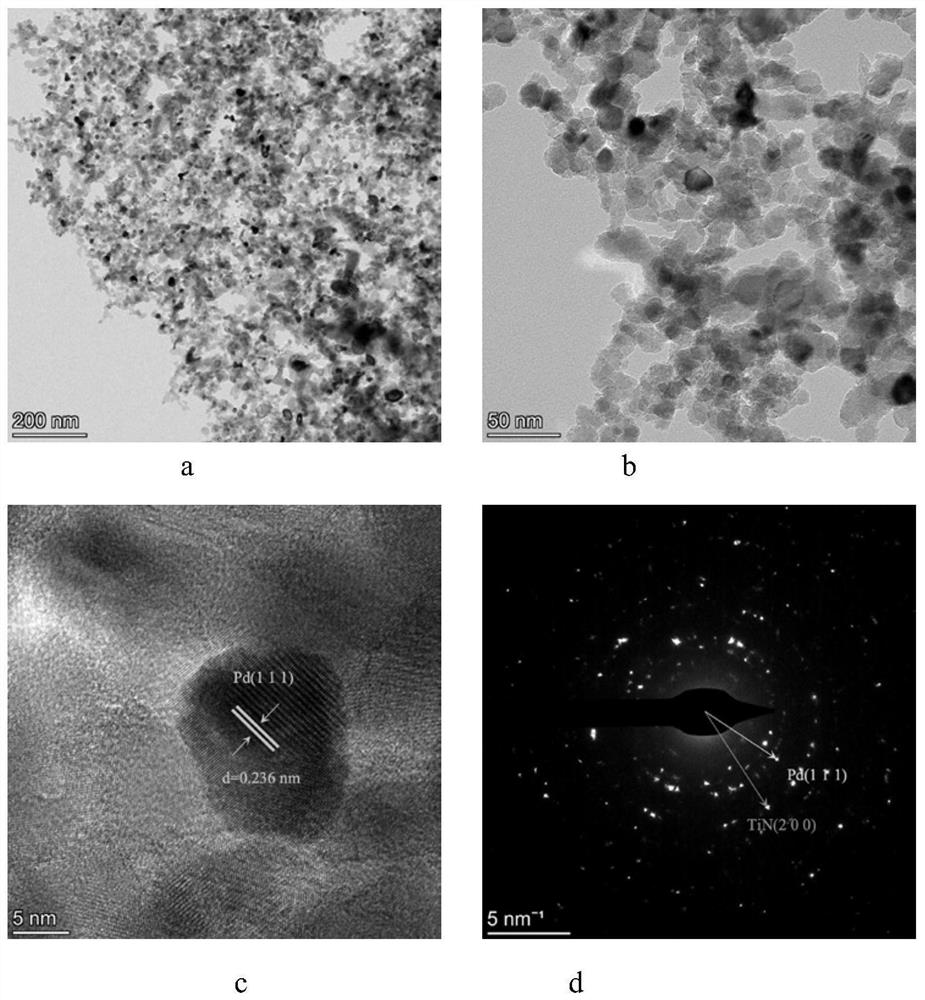
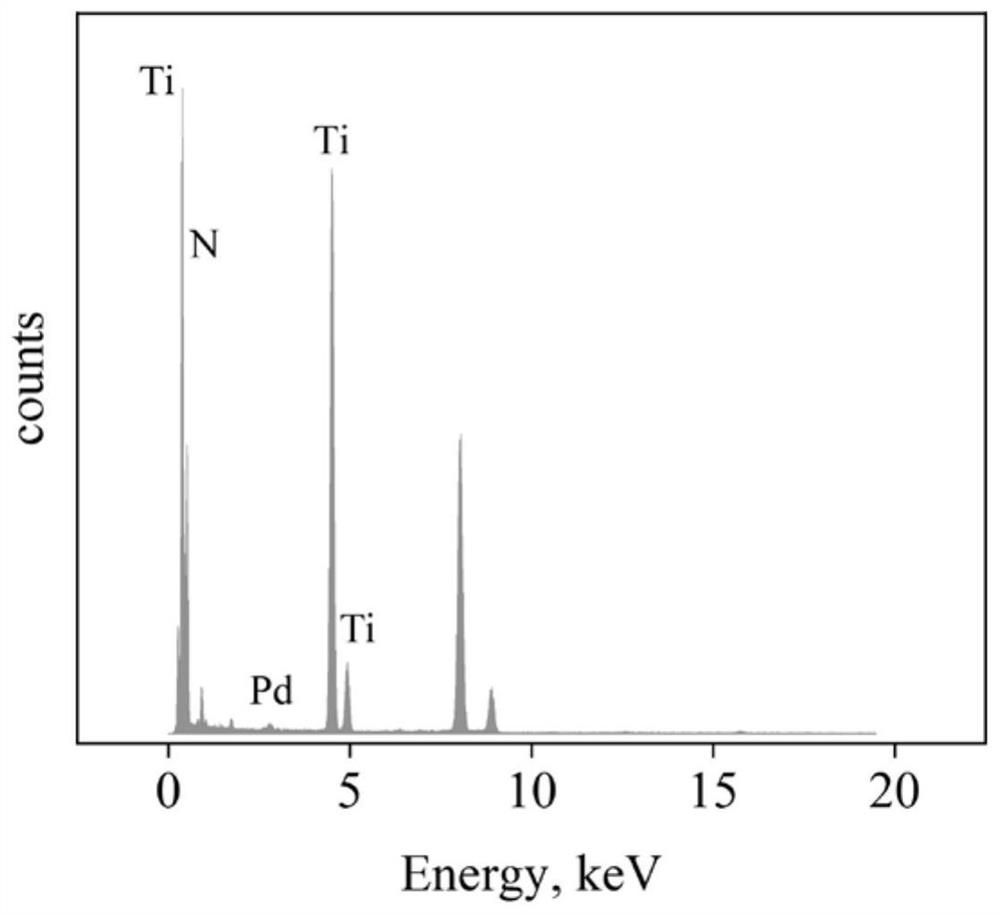
Method for degrading organic pollutants by Pd/TiN particles cooperating with electrochemistry
An organic pollutant and electrochemical technology, applied in chemical instruments and methods, water pollutants, water/sewage treatment, etc., can solve the problems of high energy consumption and poor degradation effect, and achieve the effect of improving the degradation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1, adopt NaBH 4 Preparation of Pd / TiN Particles by Reduction Process
[0029] 0.177g (0.001mol) PdCl 2 The powder was stirred and dissolved in 1000 mL of 0.1755 g / L (containing NaCl 0.003 mol) NaCl aqueous solution at a molar ratio of 1:3 to obtain 1 mM PdCl 2 solution as a Pd precursor. Weigh 495mg (0.008mol) of TiN into a 250mL three-neck flask, then add 50mL of ultrapure water, ultrasonically in an ultrasonic cleaner for 10min to disperse the powder evenly, then add 50mL of PdCl 2 solution, and stirred under 300r / min mechanical stirring for 1h, and adjusted the pH to 10 with 1M NaOH aqueous solution. Then add 10mL 1M NaBH dropwise 4 Aqueous solution, continuously stirred for 3h, after the stirring was completed, the mixture was filtered, the filter cake was washed with ultrapure water to neutrality (pH 7), and then dried under vacuum at 60°C for 12h to finally obtain Pd with a mass loading of 1%. / TiN particles 0.5 g. In order to prove that the cata...
Embodiment 2
[0030] The impact of embodiment 2 different degradation modes on the degradation of diclofenac sodium
[0031] 1. Electrolytic reaction
[0032] Carried out in a 250mL cylindrical single-chamber electrolyzer, with Ti 4 o 7 An electrode (1.5cm*2cm, purchased from Changsha Purong Chemical Co., Ltd.) was used as the anode, a Ti sheet (1.5cm*2cm) was used as the cathode, and the distance between the electrodes was fixed at 1cm. Take 200ml of electrolyte and add it to the electrolytic cell, and apply 10mA / cm on the anode 2 The DC current is supplied by a DC digital power supply. Electrolysis reaction at 25°C for 2h. Every 30min, adopt sampling needle to get 1.5ml sample from reaction solution, filter by 0.22 μ m polypropylene membrane and measure sample peak area by high performance liquid chromatography (HPLC), obtain diclofenac sodium concentration in the sample according to diclofenac sodium standard curve, and then Calculate the degradation rate of diclofenac sodium.
[0...
Embodiment 3
[0043] Example 3 Effects of different Pd / TiN dosages on the degradation of diclofenac sodium
[0044] Change the dosage of Pd / TiN in the electrochemical-Pd / TiN-PDS system in Example 1 to 0.05, 0.1, 0.25, 0.5 g / L respectively, and other operations are the same as in Example 1. The experimental results are shown in Table 1. As the dosage of Pd / TiN increased from 0.05g / L to 0.25g / L, the degradation rates of diclofenac sodium were 77.5%, 94.3% and 97.3%, respectively. When Pd / TiN When the dosage of diclofenac sodium was further increased to 0.5g / L, the degradation rate of diclofenac sodium decreased to 52.5%. Among them, when the dosage of Pd / TiN is 0.1, 0.25g / L, the degradation rate of diclofenac sodium is 94.3% and 97.3%, respectively, the difference is not big, so we choose the low dosage of 0.1g / L is the best value.
[0045] Table 1. Effects of different Pd / TiN dosages on the degradation of diclofenac sodium
[0046] Dosage (g / L) 0.05 0.1 0.25 0.5 Degrada...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com