Method for analyzing related substances of pidotimod oral solution
A technology for related substances and oral solutions, which is applied in the field of determination of drug-related substances, can solve the problems of low detection ability, insufficient control of impurities, low proportion of organic phase, etc., and achieve good repeatability, strong specificity and high accuracy. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In this example, the solution containing pidotimod is taken as an example, and the methodological validation of the chromatographic method is used.
[0022] 1. Related substance location test
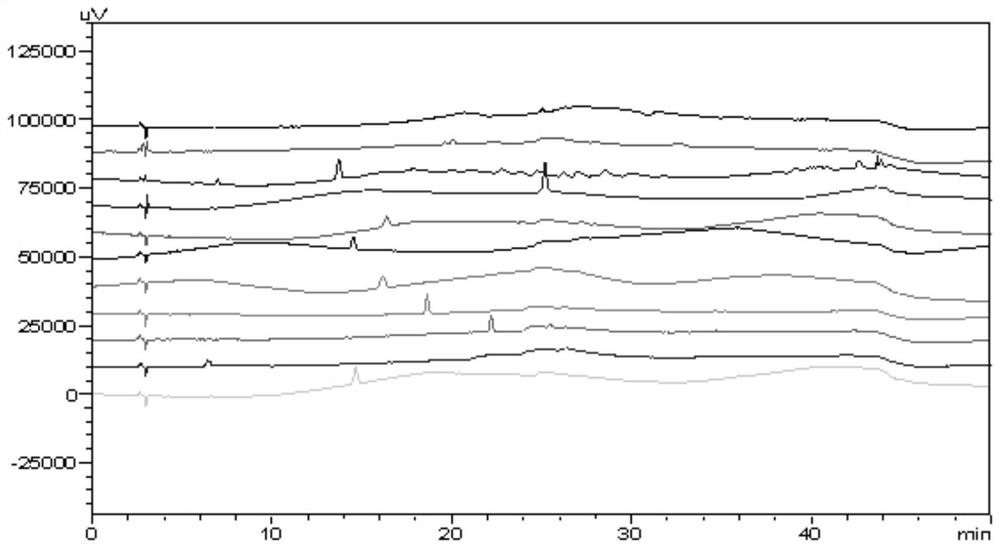
[0023] Prepare the sample solution of pidotimod, the control solution of each related substance, and the mixed solution of pidotimod and each related substance, inject them into the liquid chromatograph respectively, and record the chromatogram. The results are shown in Table 1, and the spectrum is shown in figure 1 .
[0024] Table 1 Pidotimod and the results of the separation parameters of each impurity
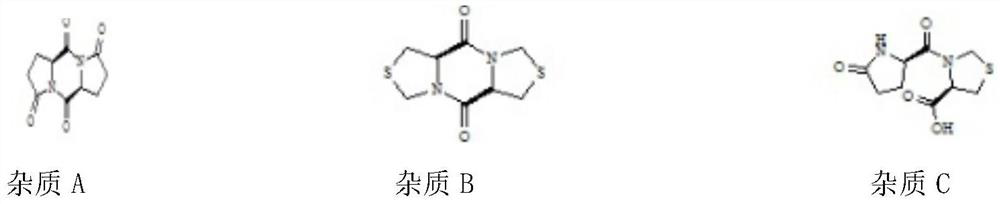
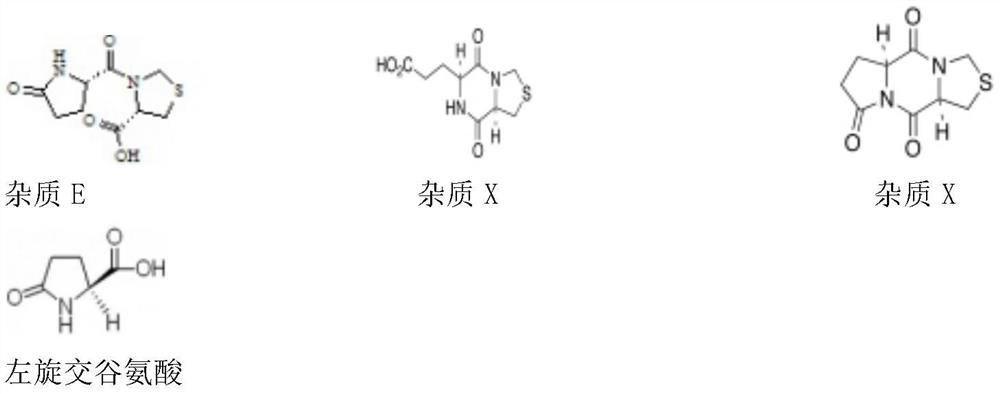
[0025] Impurity name Individual injection retention time Mixed injection retention time relative retention time Separation from front peak L-pyroglutamic acid 6.435 5.805 0.40 / Impurity A 13.719 12.948 0.89 17.844 Pidotimod 14.660 14.517 1.00 3.802 Impurity C 16.396 16.246 1.12 3.538 Impurity E 16.177 16.246 1.12...
Embodiment 2
[0048] In this embodiment, six batches of pidotimod oral solution pilot drug were taken as an example to determine related substances.
[0049] Accurately measure 1ml each of 6 batches of pidotimod oral solution, place them in 100ml measuring bottles respectively, and use pH6.8 phosphate buffer (take 1.3g of potassium dihydrogen phosphate, 1.55g of disodium hydrogen phosphate (anhydrous substance) , dissolved in water and diluted to 1000ml, adjusted to pH 6.80 with phosphoric acid) diluted to the mark, shaken up to obtain the test solution; take pidotimod reference substance, L-pyroglutamic acid reference substance, impurity X reference substance and impurity An appropriate amount of each Y reference substance was dissolved and diluted with pH 6.8 phosphate buffer to make a solution containing about 2.5 μg of each impurity per 1 ml to obtain a reference substance solution.
[0050]Chromatographic conditions: use Shimadzu high-performance liquid chromatography, ultraviolet dete...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com