Quantitative determination method for specific IgM antibody in plasma
A determination method and specific technology, which can be used in the measurement of color/spectral properties, biological testing, measuring devices, etc., can solve the problems of affecting accuracy, inconvenient detection, unavoidable non-specific binding effects, etc., and achieves low detection limit, good The effect of repeatability, excellent detection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Specific anti-Aβ in the plasma of embodiment 1 42 Quantitative determination method of oligomer IgM antibody
[0039] Method of the present invention comprises the steps:
[0040] 1. Aβ oligomer preparation: Aβ 42 Peptides, processed (to Aβ 42 Hexafluoroisopropanol (HFIP) was added to the peptide, and it was naturally volatilized in a fume hood to dry for 5-6 hours) to make it into a uniform Aβ 42 After monomer, add polymerization liquid (0.01M PBS, the NaCl of 0.085% mass volume ratio, the SDS of 0.05% mass volume ratio, medium is water, pH 7.2) makes Aβ 42 Concentration to 100M, polymerized at 4°C for 48-72h (polymerization concentration 450μg / ml).
[0041] 2. Coating: the prepared Aβ 42 The oligomers were diluted with 0.01M phosphate buffer (pH6.0), added to the microtiter plate, and coated overnight at 4°C.
[0042]3. Sealing: Pour off the microplate liquid and wash the plate 5 times with the plate washing solution. Add 200 μl / well blocking solution, block at...
experiment example 1
[0052] Experimental Example 1Aβ 42 Optimization of co-incubation conditions with plasma
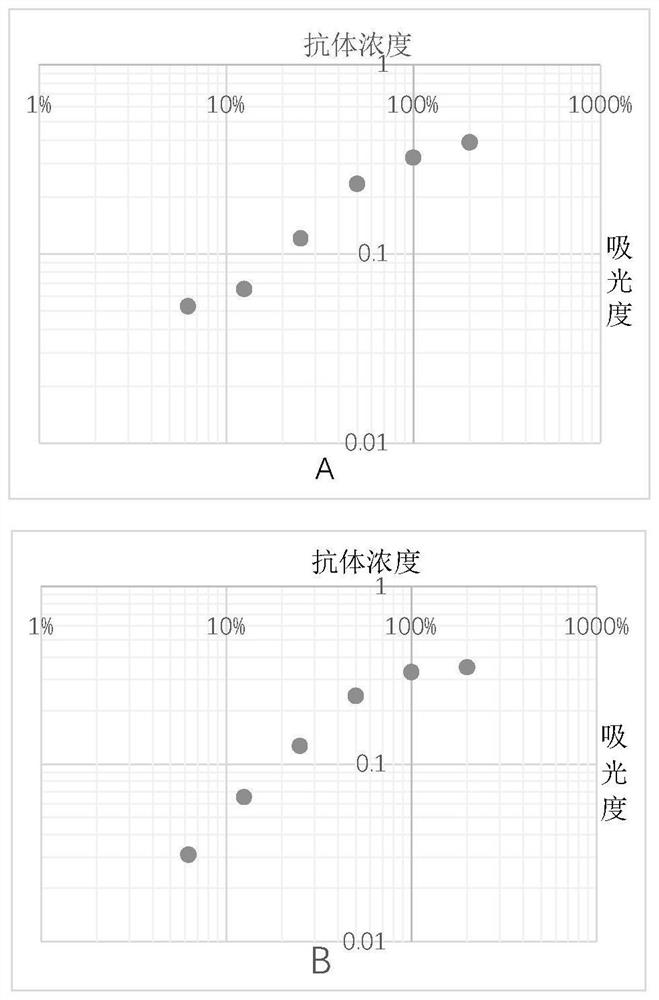
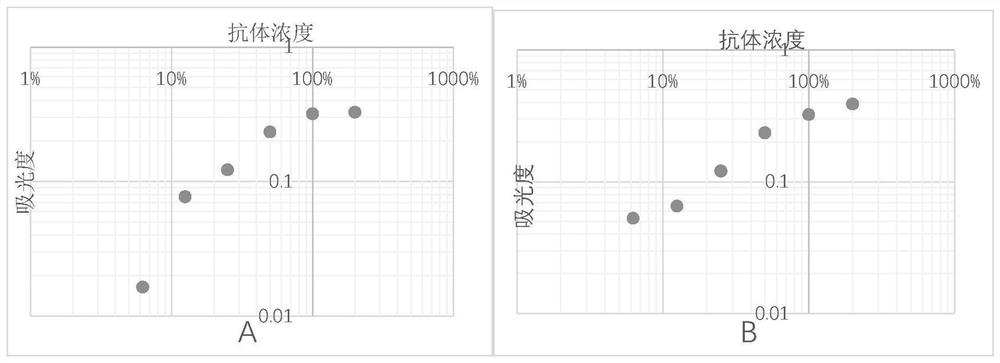
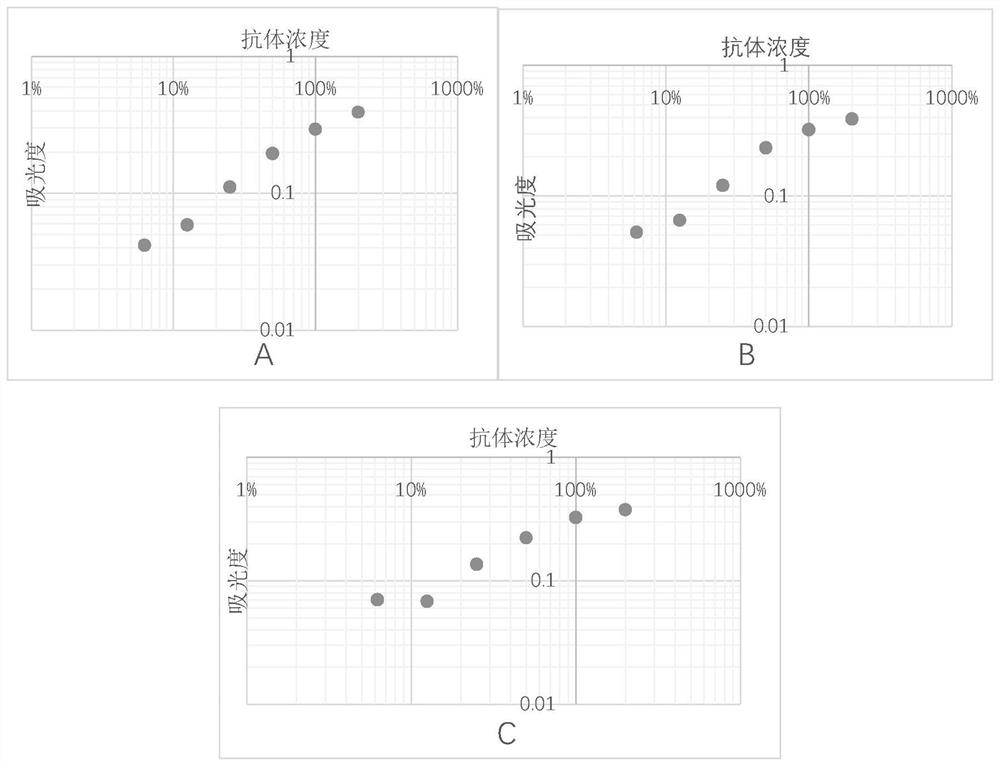
[0053] After diluting the mixed plasma of thousands of people to different concentrations, each concentration is divided into 2 parts, one of which is the standard plasma, and the other is treated with Aβ 42 As a control plasma after incubation, for Aβ 42 Co-incubation conditions with plasma were optimized (unmentioned Aβ 42 The antibody detection steps are the same as in Example 1), and the optimization effect is judged by the standard curve obtained from the final detection value of the standard plasma.
[0054] Condition 1: different Aβ 42 Co-incubation of species and plasma: Dilute 20 times, 40 times, 80 times, 160 times, 320 times, 640 times plasma respectively with Aβ 42 Oligomers and Aβ 42 Monomers were incubated in pH 8.8 buffer at 37°C for 3h. Use it as a control plasma to detect Aβ in plasma 42 IgM antibody content, the absorbance measured with different dilutions of pla...
experiment example 2
[0068] Experimental example 2 methodological verification of the present invention
[0069] This experimental example carries out methodological evaluation to the method of embodiment 1, specifically as follows:
[0070] 1. Method detection limit: The blank control was measured 8 times, and the OD values were 0.097, 0.102, 0.099, 0.095, 0.102, 0.097, 0.102, 0.097, and the calculated standard deviation δ was 0.0028, which was brought into the formula (LOD=3.3*δ / standard curve slope) the calculated LOD value is 0.0146. Then bring it into the marking line to calculate the detection limit of the method: 2.07%.
[0071] 2. Method repeatability: Dilute a plasma sample to be tested to three different concentrations: 50-fold dilution, 100-fold dilution, and 200-fold dilution. Each concentration is tested in three repetitions, and the CV value is calculated. The measured results were 91.14%, 95.68%, 99.029%, 42.46%, 46.54%, 42.15%, 23.20%, 23.98%, 23.98%, respectively. After conv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com