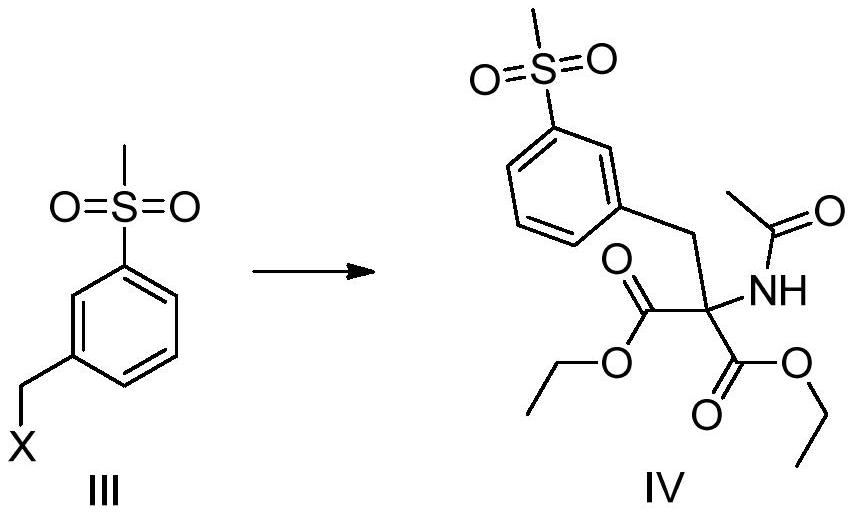
Lifitegrast intermediate and preparation method thereof
A technology for rifiplast and intermediates, applied in the field of rifiplast intermediates and their preparation, can solve the problems of unsuitability for industrialization and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
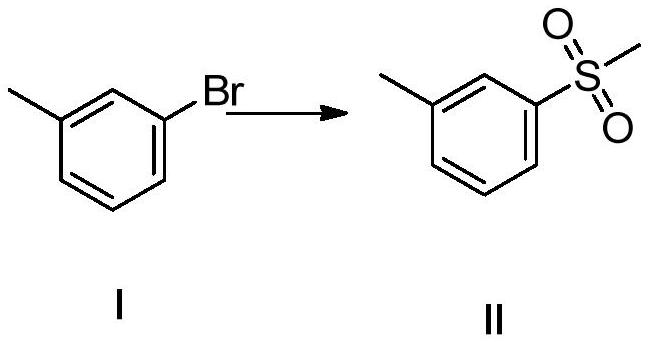
[0047] Embodiment 1: the synthesis of intermediate formula II:
[0048] A 2L three-necked flask was successively added m-bromobenzenetoluene (85.5g, 0.5mol, 1eq), sodium methanesulfonate (61.2g, 0.6mol, 0.6eq), cuprous iodide (4.5g), sodium tert-butoxide ( 72g, 0.75mol, 1.5eq), 800ml dry DMF, nitrogen protection, react overnight at 110°C, TLC control, the reaction is complete; post-treatment: the reaction solution is lowered to 0-5°C, add 600ml ice water, add ethyl acetate (300ml*3), the organic phase was combined, the organic phase was extracted with 500ml water and 500ml saturated sodium chloride, the organic phase was dried, filtered, and concentrated under reduced pressure to obtain an oil, which was recrystallized with petroleum ether to obtain 77g of a yellow solid, Yield 90%, purity 98.5%.
Embodiment 2
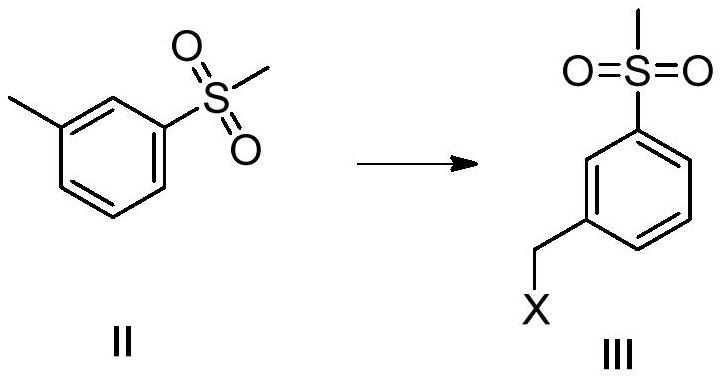
[0049] Embodiment 2: the synthesis of intermediate formula III:
[0050] A 2L three-neck flask was sequentially added the formula II (85g, 0.5mol, 1eq) obtained in Example 1, 600ml of chloroform, started stirring, and added NBS (106.8g, 0.6mol, 1.2eq) and AIBN (8.2g, 0.05mol, 0.1 eq), 20-25°C, stirring for 5h, controlled by TLC, after the reaction is complete, post-treatment: add 300ml of 10% sodium bisulfite, extract the organic phase with 300ml of water and saturated sodium chloride, dry the organic phase, and filter , and concentrated to obtain 112.05 g of a yellow oil, with a yield of 90% and a purity of 95.4%.
Embodiment 3
[0051] Embodiment 3: the synthesis of intermediate formula III:
[0052] The difference between this embodiment and Example 2 is that the halogenation reagent is selected from NCS, the catalyst is selected from BPO, and the organic solvent A is selected from carbon tetrachloride; other reaction conditions are the same as in Example 2; the yield of the product is 86%, and the purity is 98.60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com