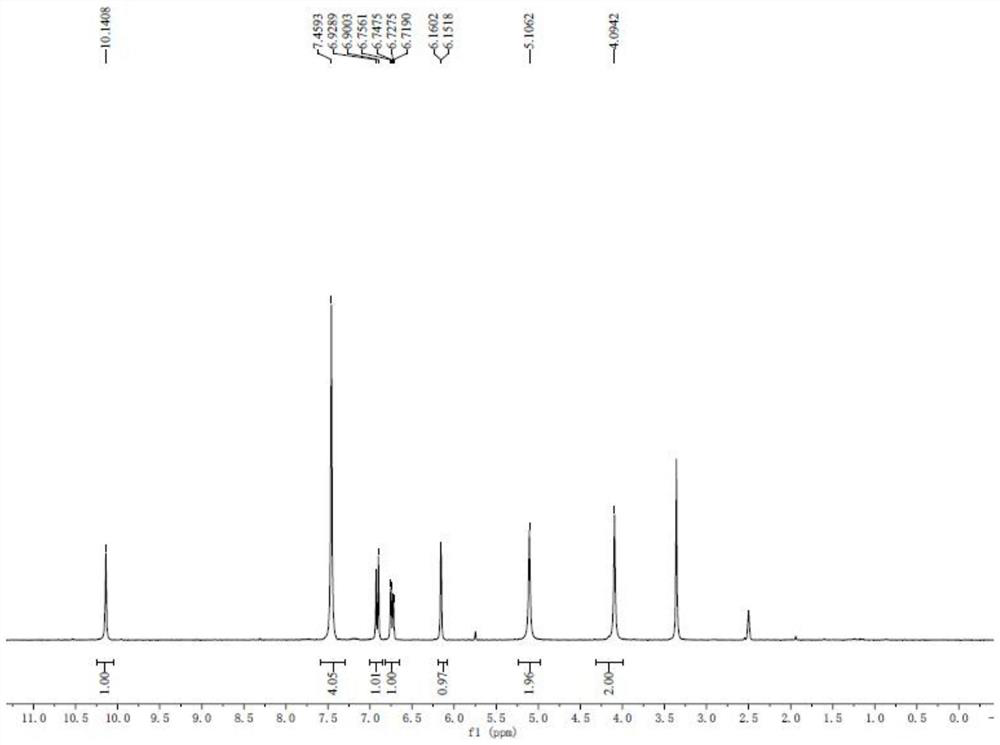
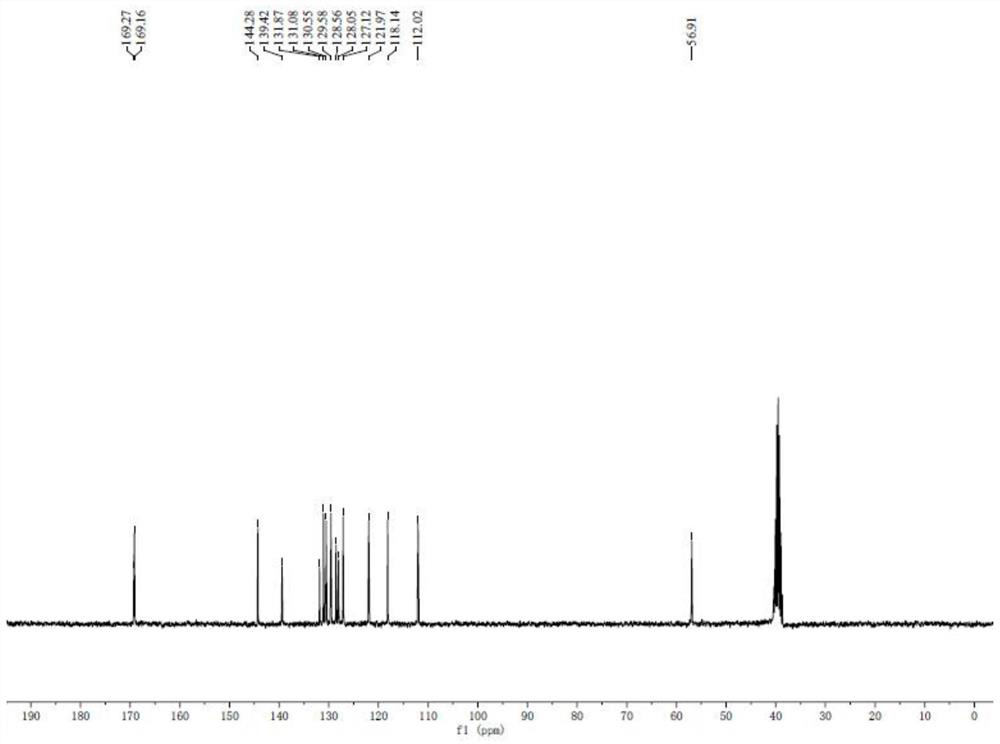
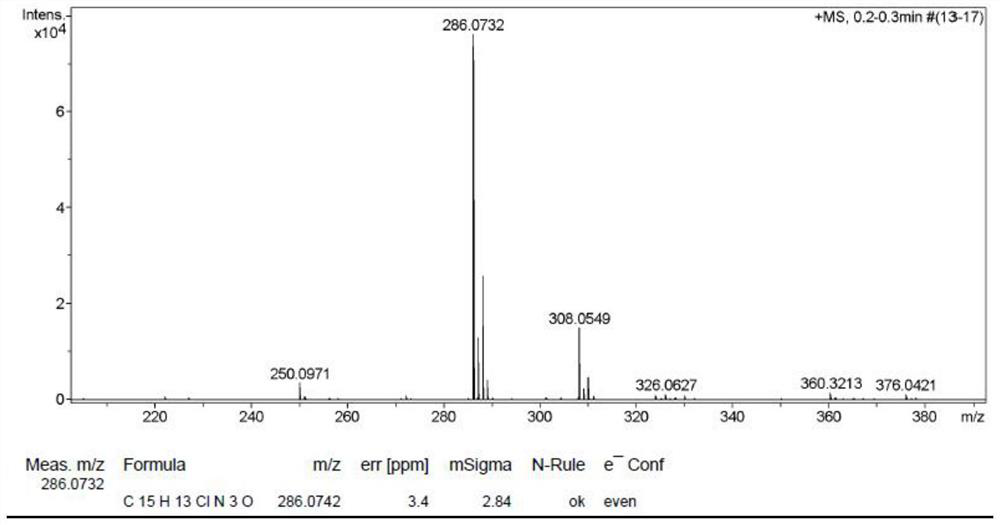
Novel method for preparing 7-amino clonazepam compound
A technology of aminochloride and compounds, applied in the direction of organic chemistry, etc., can solve problems such as troublesome disposal and large environmental impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A method for synthesizing 7-aminoclonazepam compound, comprising the following steps:
[0044] (1) Synthesis of intermediate I:
[0045]
[0046] Weigh 2-amino-4-nitrobenzoic acid (5.46g, 30mmol) and transfer it to a 250mL flask, add 80mL of acetic anhydride, then heat to reflux and react for 12h. Cool to room temperature, filter, and rinse the obtained solid with a petroleum ether-ethyl acetate mixture to obtain 5.75 g of intermediate I as a light yellow solid, with a yield of 93%;
[0047] (2) Synthesis of Intermediate II:
[0048]
[0049] Weigh intermediate I (4.12g, 20mmol), dissolve it in 80mL, add 2-chlorophenylmagnesium bromide (40mmol, 1mol / L tetrahydrofuran solution) dropwise under ice-water bath, remove the ice-water bath after 20 minutes , after the reaction is complete, add 30 mL of saturated ammonium chloride solution to quench the reaction, separate the layers, collect the organic phase, extract the water phase (30 mL*2), combine the organic phase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com