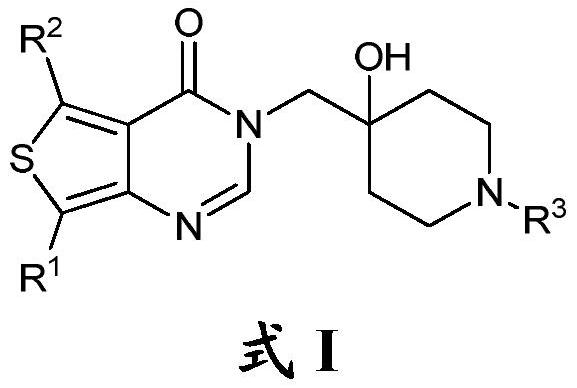
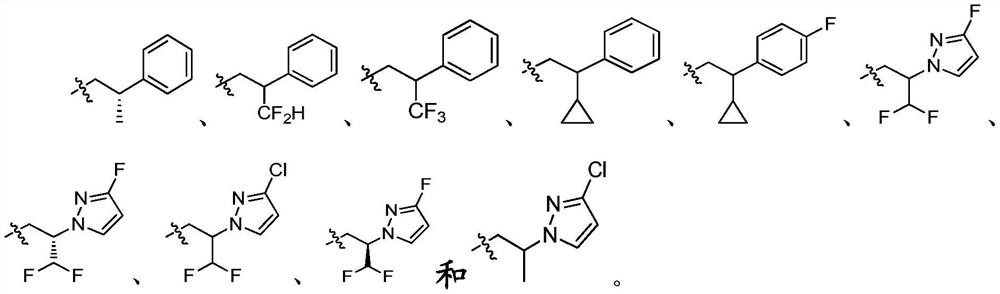
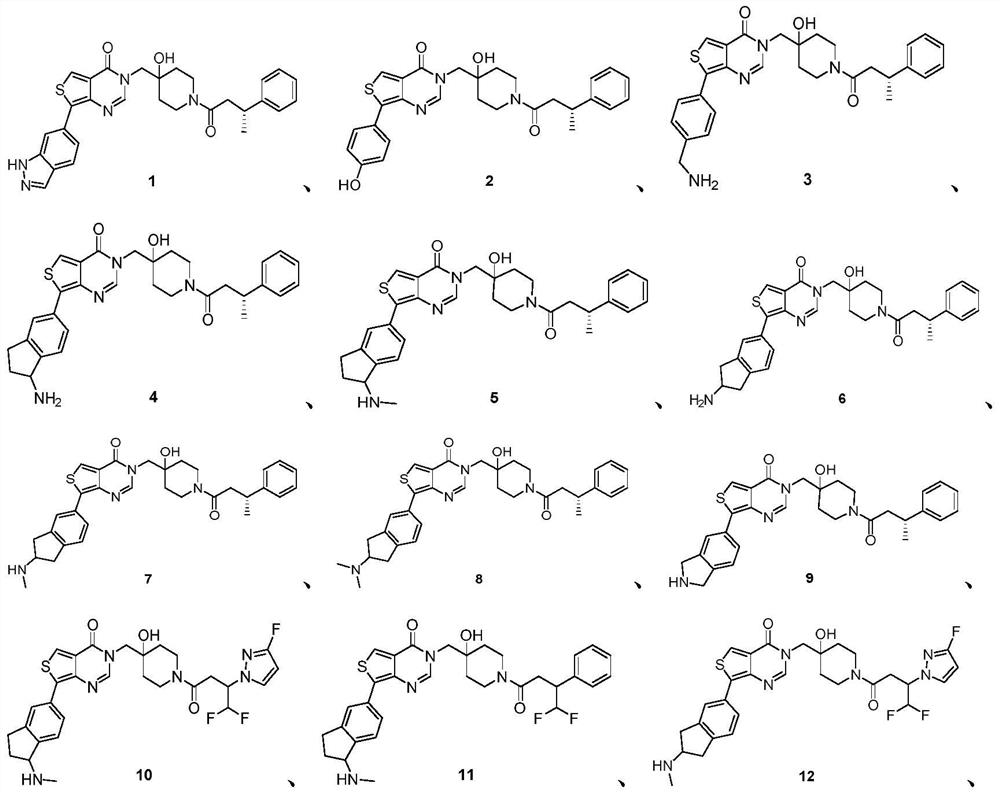
Thienopyrimidinone compound and medical application thereof
A technology of compounds and medicinal salts, applied in the fields of pharmaceutical formulations, medical preparations containing active ingredients, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0301] Embodiment one (R)-3-((4-Hydroxy-1-(3-phenylbutyryl)piperidin-4-yl)methyl)-7-(1H-indazole-6- base) Thieno[3,4-d]pyrimidin-4(3H)-one (compound 1)
[0302]
[0303] Step 1: (R)-7-bromo-3-((4-hydroxy-1-(3-phenylbutyryl)piperidin-4-yl)methyl)thieno[3,4-d]pyrimidine- Synthesis of 4(3H)-ketone (compound 1-2)
[0304] At room temperature, compound 1-1 (0.13g, 0.56mmol), (R)-3-phenyl-1-(1-oxa-6-aza-spiro[2.5]oct-6-yl)butanone (Synthesis method reference WO2018073602) (0.15g, 0.56mmol) was dissolved in DMF (4mL), potassium carbonate (0.16g, 1.13mmol) was added, and the reaction solution was heated to 80°C for 8h. The reaction mixture was cooled to room temperature, separated and purified by preparative high performance liquid chromatography (method D), and lyophilized to obtain the title compound, 0.14 g. ESI-MS(m / z):490.1,492.1[M+H] + .
[0305] Step 2: (R)-3-((4-Hydroxy-1-(3-phenylbutyryl)piperidin-4-yl)methyl)-7-(1H-indazol-6-yl)thieno Synthesis of [3,4-d]pyrimid...
Embodiment 2
[0309] Embodiment two (R)-3-((4-Hydroxy-1-(3-phenylbutyryl)piperidin-4-yl)methyl)-7-(4-hydroxybenzene base) Thieno[3,4-d]pyrimidin-4(3H)-one (compound 2)
[0310]
[0311] At room temperature, compound 1-2 (30mg, 0.06mmol), 4-hydroxyphenylboronic acid (20mg, 0.09mmol), potassium carbonate (25mg, 0.18mmol) were added to the reaction flask in turn, and then 1,4-diox Hexacyclic (4 mL) and water (0.8 mL). Finally, palladium tetrakistriphenylphosphine (7.1 mg, 0.006 mmol) was added, and nitrogen was replaced three times. Under the protection of nitrogen, it was heated to 80°C and stirred for 4h. The reaction solution was cooled to room temperature, concentrated under reduced pressure, purified by preparative high performance liquid chromatography (method D), and lyophilized to give the title compound, 20 mg.
[0312] Its structure is characterized as follows:
[0313] 1 H NMR (400MHz, DMSO-d 6 )δ9.74(s,1H),8.34(s,1H),7.97(d,J=10.4Hz,1H),7.91-7.83(m,2H),7.28-7.22(m,4H)...
Embodiment 3
[0314] Embodiment Three (R)-7-(4-(aminomethyl)phenyl)-3-((4-hydroxy-1-(3-phenylbutyryl)piperidine-4- Synthesis of yl)methyl)thieno[3,4-d]pyrimidin-4(3H)-one (compound 3)
[0315]
[0316]Step 1: (R)-4-(3-((4-Hydroxy-1-(3-phenylbutyryl)piperidin-4-yl)methyl-4-oxo-3,4-dihydrothiophene Synthesis of tert-butyl[3,4-d]pyrimidin-7-yl)benzylcarbamate (compound 3-1)
[0317] According to the operation described in Example 2, compound 1-2 (20mg, 0.04mmol) and 4-(N-tert-butoxycarbonylaminomethyl)phenylboronic acid (15mg, 0.06mmol) were used as the reaction raw materials to replace compound 1 respectively -2 (30mg, 0.06mmol) was reacted with 4-hydroxyphenylboronic acid, and the reaction solution was concentrated under reduced pressure to obtain the crude product of the title compound, 21mg, which was directly used in the next reaction. ESI-MS(m / z):617.3[M+H] + .
[0318] Step 2: (R)-7-(4-(aminomethyl)phenyl)-3-((4-hydroxy-1-(3-phenylbutyryl)piperidin-4-yl)methyl)thiophene Syn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com