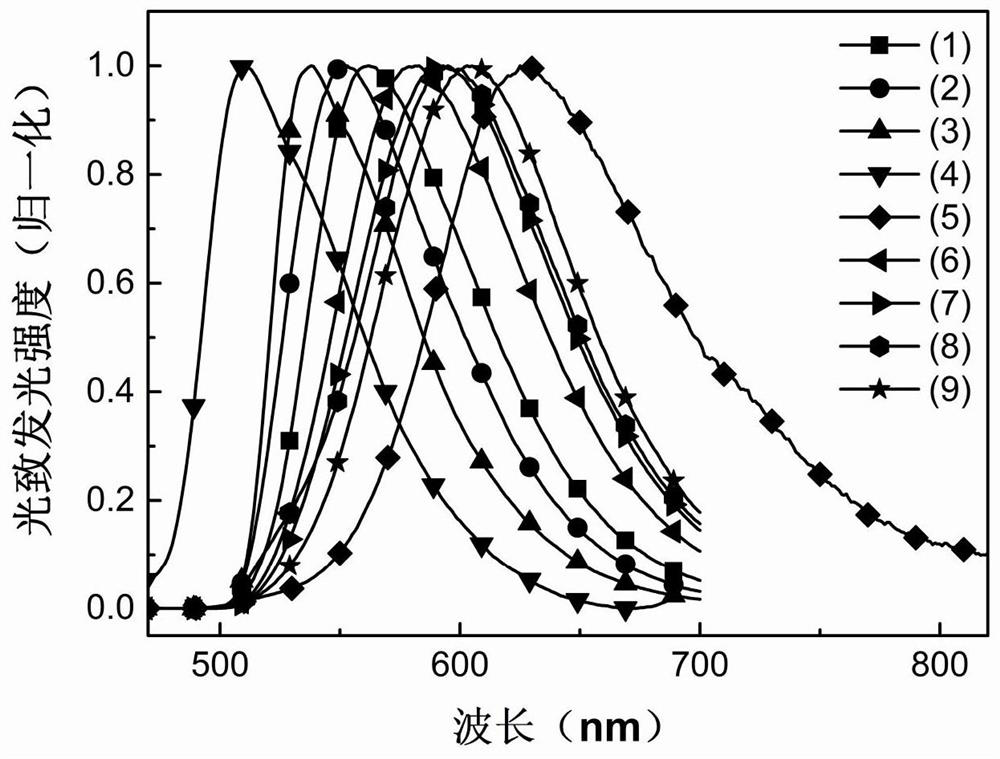
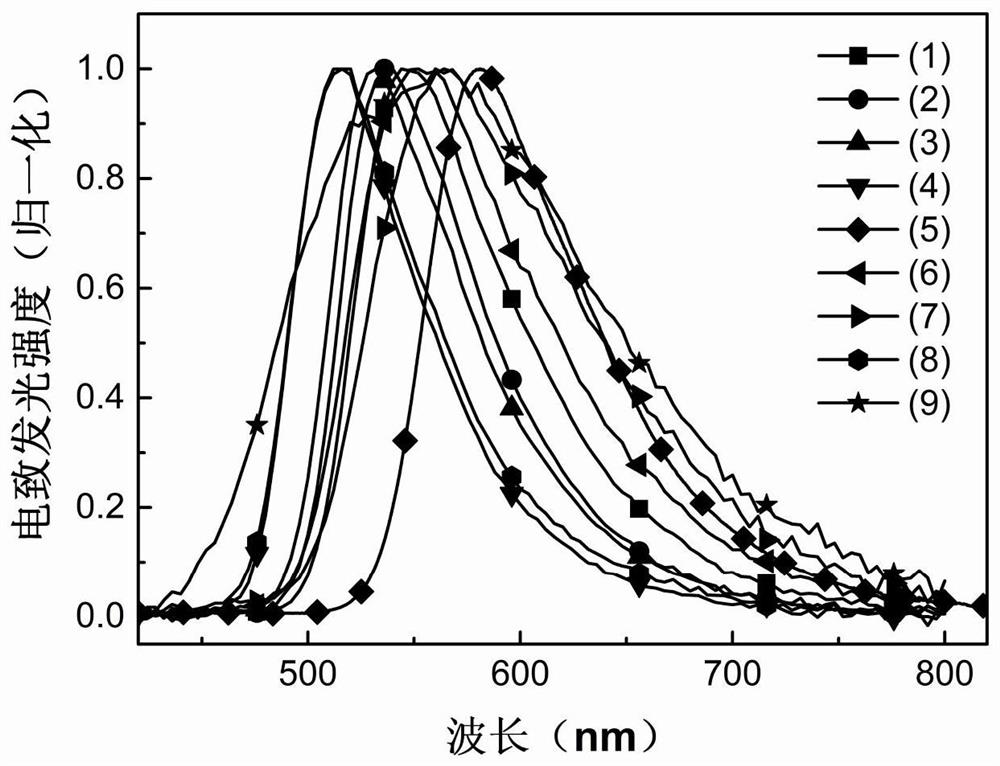
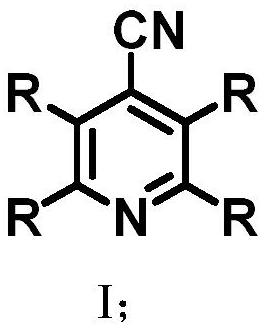
Thermally activated delayed fluorescent material based on cyanopyridine and preparation method and application thereof in organic electroluminescence
A technology of thermally activated delayed and fluorescent materials, applied in the fields of luminescent materials, organic chemistry, chemical instruments and methods, etc., to achieve good device performance, achieve device performance, and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Compound 1
[0040] 2,3,5,6-tetrafluoro-4-cyanopyridine (0.5g, 2.84mmol), 3,6-dimethylcarbazole (2.77g, 14.20mmol), potassium carbonate (3.92g, 28.40mmol) , DMSO 12mL, heated at 150°C, and reacted at normal pressure under nitrogen atmosphere for 18h. After the reaction solution was cooled to room temperature, it was poured into 400 mL of water and stirred. A large amount of solids precipitated. After suction filtration, a solvent was prepared with dichloromethane and petroleum ether at a volume ratio of 1:2, and the final product was purified by silica gel column chromatography. 1 H NMR (400MHz, CDCl 3 ,δppm): 7.59(s, 4H), 7.51(s, 4H), 7.33-7.30(d, J=12Hz, 4H), 7.18-7.16(d, J=8Hz, 4H), 7.02-7.00(m, 4H), 6.88-6.85 (m, 4H), 2.42-2.34 (m, 24H).
[0041]
Embodiment 2
[0042] Example 2: Compound 2
[0043] 2,3,5,6-tetrafluoro-4-cyanopyridine (0.5g, 2.84mmol), 3,6-di-tert-butylcarbazole (3.97g, 14.20mmol), potassium carbonate (3.92g, 28.40mmol ), DMSO 12mL, heated at 150°C, and reacted at normal pressure under nitrogen atmosphere for 18h. After the reaction solution was cooled to room temperature, it was poured into 400 mL of water and stirred. A large amount of solids precipitated. After suction filtration, a solvent was prepared with dichloromethane and petroleum ether at a volume ratio of 1:3, and the final product was purified by silica gel column chromatography. 1 H NMR (400MHz, CDCl 3 , δppm): 7.61-7.60(d, J=4Hz, 4H), 7.55-7.54(d, J=4Hz, 4H), 7.15-7.13(d, J=8Hz, 4H), 7.04-7.01(m, 4H ), 6.97-6.95 (d, J=8Hz, 4H), 6.90-6.87 (m, 4H), 1.37-1.34 (d, J=12Hz, 72H).
[0044]
Embodiment 3
[0045]Example 3: Compound 3
[0046] 2,3,5,6-tetrafluoro-4-cyanopyridine (0.5g, 2.84mmol), 2,7-dimethylcarbazole (2.77g, 14.20mmol), potassium carbonate (3.92g, 28.40mmol) , DMSO 12mL, heated at 150°C, and reacted at normal pressure under nitrogen atmosphere for 18h. After the reaction solution was cooled to room temperature, it was poured into 400 mL of water and stirred. A large amount of solids precipitated. After suction filtration, a solvent was prepared with dichloromethane and petroleum ether at a volume ratio of 1:2, and the final product was purified by silica gel column chromatography. 1 H NMR (400MHz, CDCl 3 , δppm): 7.61-7.59(d, J=8Hz, 4H), 7.55-7.53(d, J=8Hz, 4H), 7.26(s, 4H), 7.09(s, 4H), 6.94-6.92(d, J=8Hz, 4H), 6.89-6.87 (d, J=8Hz, 4H), 2.23-2.17 (d, J=24Hz, 24H).
[0047]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com