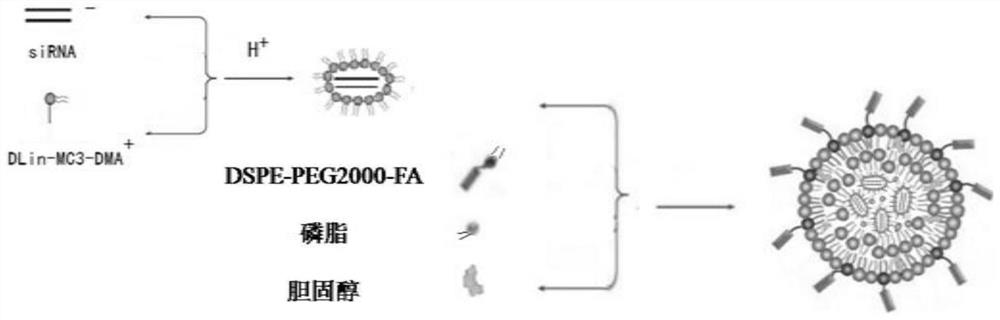
SiRNA-loaded nano-liposome hybrid micelle and preparation method and application thereof
A kind of micellar and nanotechnology, applied in the direction of pharmaceutical formula, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of no treatment method, etc., and achieve the effect of simple steps and lower concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Preparation of siRNA-loaded nano-lipid hybrid micelles
[0053] 1) Preparation of siRNA@DLin-MC3-DMA complex:
[0054] 1.1) Preparation of DLin-MC3-DMA solution: Weigh 0.5mg DLin-MC3-DMA, add 500μL of absolute ethanol to it, fully dissolve to obtain 1mg / mL DLin-MC3-DMA solution, store at -20°C , and set aside; take 200μLDLin-MC3-DMA solution and add absolute ethanol to dilute to 2mL, mix thoroughly with ultrasound, place in a round-bottomed flask, place in a constant temperature water bath at 37°C, remove the solvent by rotary evaporation for 15min, add 4mL of phosphate buffer, Hydrate at 37°C for 20 minutes and sonicate for 5 minutes to obtain a 0.1 mg / mL DLin-MC3-DMA micellar solution, store at 4°C for later use.
[0055] 1.2) Preparation of siRNA solution: Take 1OD siRNA and centrifuge at 4000rpm / min for 3min, then slowly open the cap of the tube, add 125μL of DEPC water, shake and dissolve to obtain a 20μM siRNA solution, and then divide the siRNA solution in a ste...
experiment example 1
[0069] Characterization of physicochemical properties of siRNA-loaded nanolipid hybrid micelles
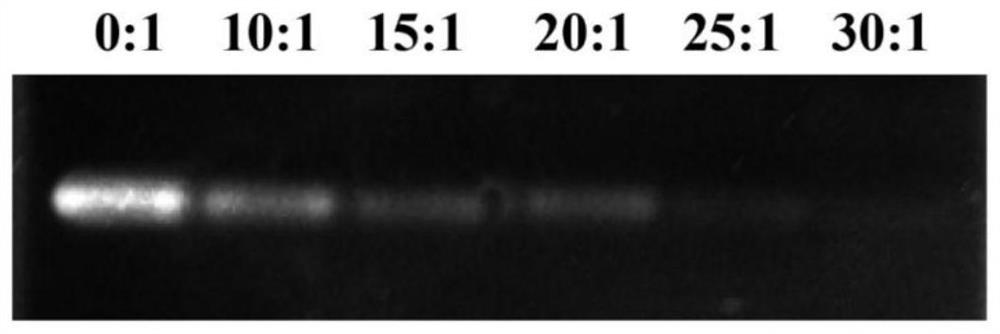
[0070] 1.1 Agarose gel electrophoresis retardation of siRNAs@DLin-MC3-DMA complex
[0071] The preparation process of agarose gel is as follows: accurately weigh 1.2g of agarose in a conical flask, add 40mL of electrophoresis buffer (1×TBE), boil and dissolve in a microwave oven, observe that there is no insoluble matter, and pour it into the gel after cooling slightly. Insert a comb into the gel plate, let it stand at room temperature for 30 minutes, and conduct the experiment after the gel solidifies. Mix the loading buffer (5×RNA LoadingBuffer) and the sample at a ratio of 1:5 on a clean parafilm, and the sample is the prepared DLin-MC 3 - siRNA@DLin-MC with volume ratios of DMA micelles to siRNA of 30:1, 25:1, 20:1, 15:1, 10:1 3 - DMA complex and free siRNA solution to be tested.
[0072] The result is as figure 2 Shown when DLin-MC 3 -When the volume ratio of DMA to siR...
experiment example 2
[0086] 2.1 Determination of particle size and zeta potential of siRNA-loaded nanolipid hybrid micelles
[0087] 2.1.1 The effect of the addition ratio of phospholipids and cholesterol
[0088] Weigh cholesterol and phospholipids with a mass ratio of 1:4, 1:5, and 1:6, respectively, and the subsequent related steps are the same as in Example 1. After the ultrasound is completed, a blank liposome (liposome) is obtained, and 1 mL is taken to detect the particle size and potential , and the results are shown in Table 2.
[0089] Table 2
[0090] No. Cholesterol: Phospholipids Particle size / nm PDI Potential / mV 1 1:4 155.2±4.90 0.290±0.026 -4.03±1.701 2 1:5 137.1±5.90 0.259±0.034 -7.33±2.921 3 1:6 150.0±1.73 0.303±0.023 -3.19±0.587
[0091] As can be seen from Table 2, when the mass ratio of cholesterol and phospholipids measured by the Malvern particle size analyzer through dynamic light scattering is 1:5, the average particle diamete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com