Method for synthesizing uronic acid through catalytic oxidation of pyranoside
A pyranoside, catalytic oxidation technology, applied in chemical instruments and methods, sugar compounds with non-glycosyl groups, organic chemistry, etc., can solve the problems of high processing difficulty, complicated operation, human interference, etc., to improve reaction efficiency, The effect of simplifying the reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
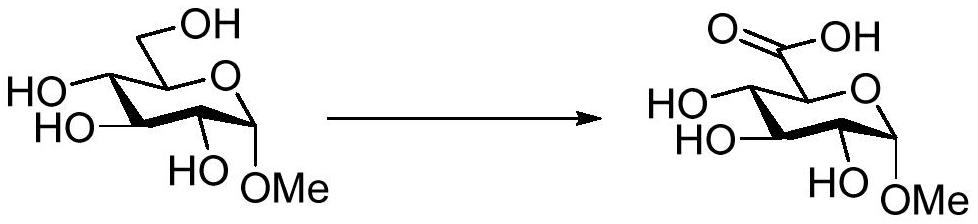
[0031] The embodiment of the present invention provides a method for catalytic oxidation of methyl-α-D-glucopyranoside to obtain methyl-α-D-glucopyranose acid. The specific reaction process is as follows:
[0032]
[0033] In a 50mL three-neck round bottom flask, add methyl-α-D-glucopyranoside 0.39g (2mmol), TEMPO 0.016g (0.1mmol) and water 10mL in sequence;
[0034] Add 0.31 g (2.2 mmol) of calcium hypochlorite solids in batches at a temperature of 0° C., and the addition time is 10 minutes. After the addition is completed, the catalytic oxidation reaction is carried out, and the reaction time is 4 hours;
[0035] After the reaction is complete, let stand to separate layers, filter under reduced pressure, adjust the pH of the obtained filtrate to 2 with 4mol / L HCl, concentrate under reduced pressure to remove solvent water, add 8 mL of methanol to the obtained concentrate, shake and filter to remove inorganic salts , the obtained filtrate was rotary evaporated to remove th...
Embodiment 2
[0038] The embodiment of the present invention provides a method for catalytic oxidation of methyl-α-D-galactopyranoside to obtain methyl-α-D-galactopyranuronic acid. The specific reaction process is as follows:
[0039]
[0040]In a 50mL three-neck round bottom flask, add methyl-α-D-galactopyranoside 0.39g (2mmol), TEMPO 0.016g (0.1mmol) and water 40mL in sequence;
[0041] Add calcium hypochlorite solid 0.43g (3mmol) in batches under the condition of 10°C, and the addition time is 10min. After the addition is completed, carry out the catalytic oxidation reaction, and the reaction time is 3h;
[0042] After the reaction is complete, let stand to separate layers, filter under reduced pressure, adjust the pH of the obtained filtrate to 3 with 3mol / L HCl, concentrate under reduced pressure to remove solvent water, add 8 mL of ethanol to the obtained concentrate, shake and filter to remove inorganic salts , the obtained filtrate was rotary evaporated to remove the solvent, and...
Embodiment 3
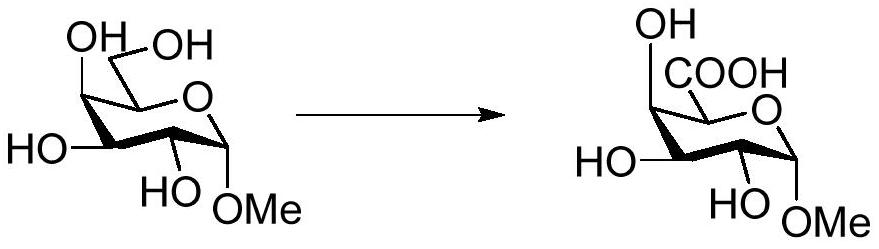
[0045] The embodiment of the present invention provides a method for catalytic oxidation of methyl-α-D-mannopyranoside to obtain methyl-α-D-mannopyranuronic acid, the specific reaction process is as follows:
[0046]
[0047] In a 50mL three-neck round bottom flask, add methyl-α-D-mannopyranoside 0.39g (2mmol), TEMPO 0.032g (0.02mmol) and water 30mL in sequence;
[0048] Add calcium hypochlorite solid 0.57g (4mmol) in batches under the condition of 20 DEG C, the feeding time is 10min, and the catalytic oxidation reaction is carried out after the feeding is completed, and the reaction time is 1h;
[0049] After the reaction is complete, let stand to separate layers, filter under reduced pressure, adjust the pH of the obtained filtrate to 2 with 5mol / L HCl, concentrate under reduced pressure to remove solvent water, add 8 mL of methanol to the obtained concentrate, shake and filter to remove inorganic salts , the obtained filtrate was rotary evaporated to remove the solvent, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com