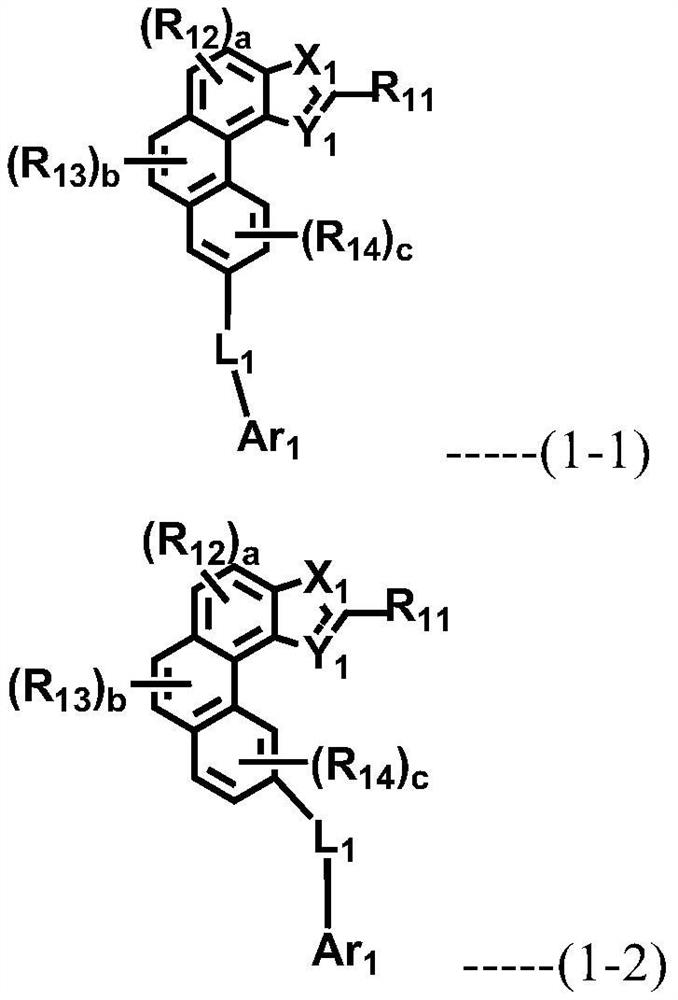
Multiple host materials and organic electroluminescent device comprising same
A host material, an unreplaced technology, applied in the field of organic electroluminescent devices, can solve the problems of not specifically disclosing a variety of host materials, and achieve the effects of good life characteristics, high luminous efficiency, and low driving voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0102] Synthesis Example 1: Preparation of Compound H1-147
[0103]
[0104] Compound A (CAS: 2085325-18-2, 4.0 g, 9.5 mmol), 2-chloro-3-phenylquinoxaline (2.8 g, 11.4 mmol), tetrakis (triphenylphosphine) palladium (0) ( Pd(PPh 3 ) 4 ) (0.5g, 0.5mmol), potassium carbonate (K 2 CO 3 ) (2.0 g, 19 mmol), toluene (30 mL), EtOH (7 mL) and water (10 mL) were added to the reaction vessel and stirred at reflux for one day. After the reaction was completed, followed by cooling to room temperature, the reaction mixture was filtered with dichloromethane (MC) through a celite filter and then distilled under reduced pressure. The residue was separated by column chromatography using dichloromethane / hexane (MC / Hex) to obtain compound H1-147 (2.7 g, yield: 57%).
[0105] compound MW melting point H1-147 499.6 266℃
Synthetic example 2
[0106] Synthesis Example 2: Preparation of Compound H1-146
[0107]
[0108] Compound A (23.8g, 56.6mmol), 2-chloro-4-(naphthalene-1-yl)-6-benzene-1,3,5-triazine (15.0g, 47.2mmol), Pd(PPh 3 ) 4 (2.72g, 2.36mmol), K 2 CO 3 (16.3 g, 118 mmol), toluene (240 mL), EtOH (60 mL) and purified water (60 mL) were added to the reaction vessel and stirred at reflux for 2 hours. After the reaction was completed, the reaction mixture was cooled to room temperature, and the organic layer was separated through a silica filter. The organic layer was distilled under reduced pressure, and recrystallized with toluene, to obtain compound H1-146 (13.8 g, yield: 51%).
[0109] compound MW melting point H1-146 576.6 231℃
Synthetic example 3
[0110] Synthesis Example 3: Preparation of Compound H1-157
[0111]
[0112] Compound A (4.0g, 9.5mmol), 2-([1,1'-biphenyl]-3-yl)-4-chloro-6-phenyl-1,3,5-triazine (3.9g , 11.4mmol), Pd(PPh 3 ) 4 (0.5g, 0.5mmol), K 2 CO 3 (2.6 g, 19 mmol), toluene (30 mL), EtOH (7 mL) and purified water (10 mL) were added to the reaction vessel and stirred at reflux for 6 hours. After the reaction was completed, followed by cooling to room temperature, the reaction mixture was stirred at room temperature, and methanol (MeOH) was then added thereto. The resulting solid was filtered under reduced pressure, and separated with MC by column chromatography to obtain compound H1-157 (4.6 g, yield: 80%).
[0113] compound MW melting point H1-157 602.7 227℃
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com