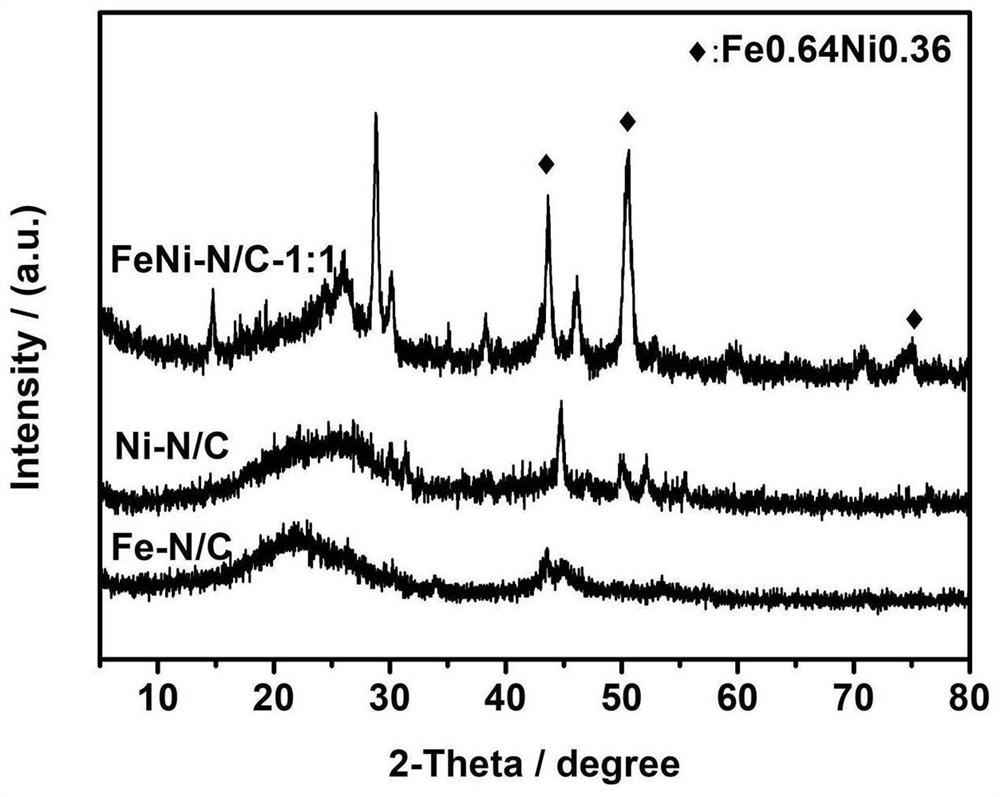
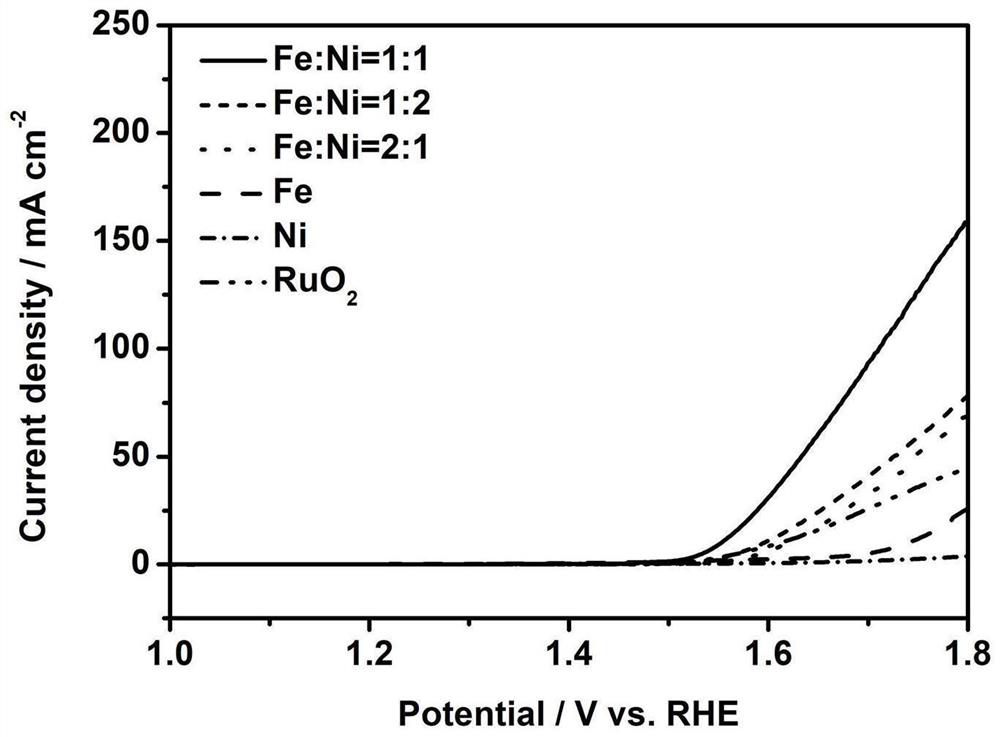
Nickel-doped iron-based bimetallic non-noble metal catalyst and preparation method thereof
A non-precious metal and catalyst technology, applied in electrodes, electrolysis process, electrolysis components, etc., can solve the problem of high overpotential of non-precious metal catalysts, and achieve the effects of low cost, excellent oxygen evolution performance and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Step 1. After mixing 250mg of SBA-15, 500mg of sucrose and 1000mg of melamine, carbonize with 580μL of concentrated sulfuric acid, then put it into a drying oven at 100°C, dry for 4-8 hours, and then dry at 160°C for 5-10 hours to obtain a carbonized masterbatch ;
[0034] Step 2. Add 500mg sucrose and 1000mg melamine to the carbonized masterbatch obtained in step 1 and stir evenly, then add 1mL 1M ferric chloride hexahydrate and 1mL 1M nickel chloride hexahydrate and mix, then carbonize with 600 μL concentrated sulfuric acid again, and stir evenly Put it into the drying oven, firstly dry at 100°C for 6 hours, and then dry at 160°C for 6 hours to obtain carbonized sub-materials;
[0035] Step 3. Put the carbonized sub-material into a magnetic boat after being ground, and under the protection of nitrogen, first sinter at a temperature of 300°C for 1 hour, then raise the temperature to 900°C and sinter for 1 hour to obtain a catalyst base material;
[0036] Step 4, pickl...
Embodiment 2
[0043] Step 1. After mixing 250mg of SBA-15, 1000mg of sucrose and 1000mg of melamine, carbonize with 650μL of concentrated sulfuric acid, then put it into a drying oven at 150°C for 7 hours, and then dry at 200°C for 5 hours to obtain a carbonized masterbatch;
[0044]Step 2. Add 1000mg sucrose and 1000mg melamine to the carbonized masterbatch obtained in step 1 and stir evenly, then add 0.5mL 1M ferric chloride hexahydrate and 1mL 1M nickel chloride hexahydrate to mix, then carbonize with 700 μL concentrated sulfuric acid again, and stir evenly Then put it into the drying oven, firstly dry at 150°C for 7 hours, and then dry at 200°C for 5 hours to obtain carbonized sub-materials;
[0045] Step 3. Put the carbonized sub-material into a magnetic boat after being ground, and under the protection of nitrogen, first sinter at a temperature of 250°C for 1.5h, then raise the temperature to 950°C and sinter for 1.5h to obtain a catalyst base material;
[0046] Step 4, pickling the c...
Embodiment 3
[0048] Step 1. After mixing 250mg of SBA-15, 1000mg of sucrose and 1000mg of melamine, carbonize with 500μL of concentrated sulfuric acid, then put it into a drying oven at 1 before 100°C, dry for 4h, and then dry at 100°C for 5h to obtain a carbonized masterbatch;
[0049] Step 2. Add 1000mg sucrose and 1000mg melamine to the carbonized masterbatch obtained in step 1, stir evenly, add 0.5mL 1M ferric chloride hexahydrate and 1mL 1M nickel chloride hexahydrate, mix, carbonize again with 540 μL concentrated sulfuric acid, stir evenly Then put it into the drying oven, firstly dry at 100°C for 4 hours, and then dry at 100°C for 5 hours to obtain carbonized sub-materials;
[0050] Step 3. Put the carbonized sub-material into a magnetic boat after being ground, and under the protection of nitrogen, first sinter at a temperature of 200°C for 1 hour, then raise the temperature to 700°C and sinter for 0.5h to obtain a catalyst base material;
[0051] Step 4, pickling the catalyst base...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com