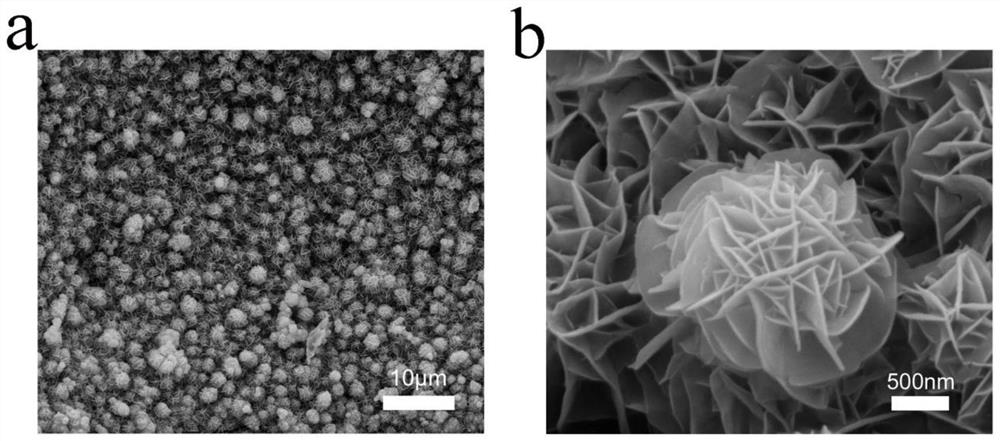
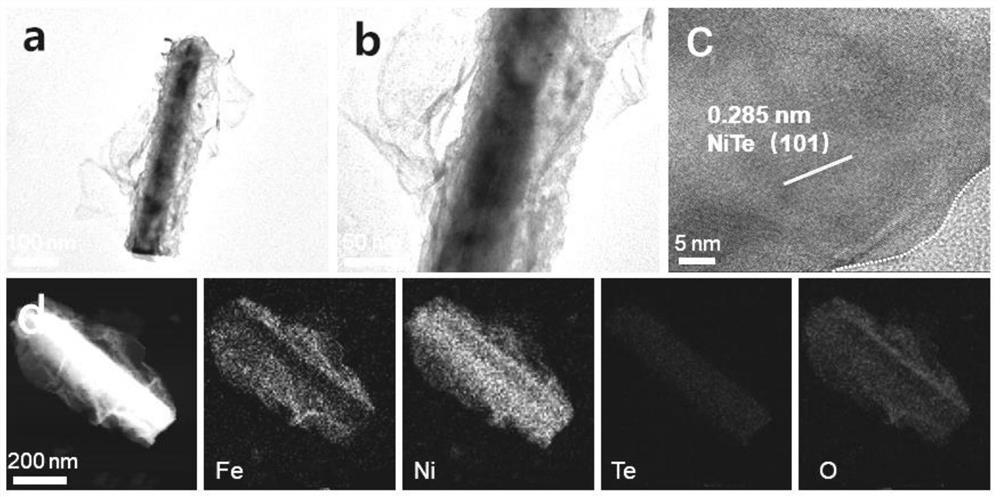
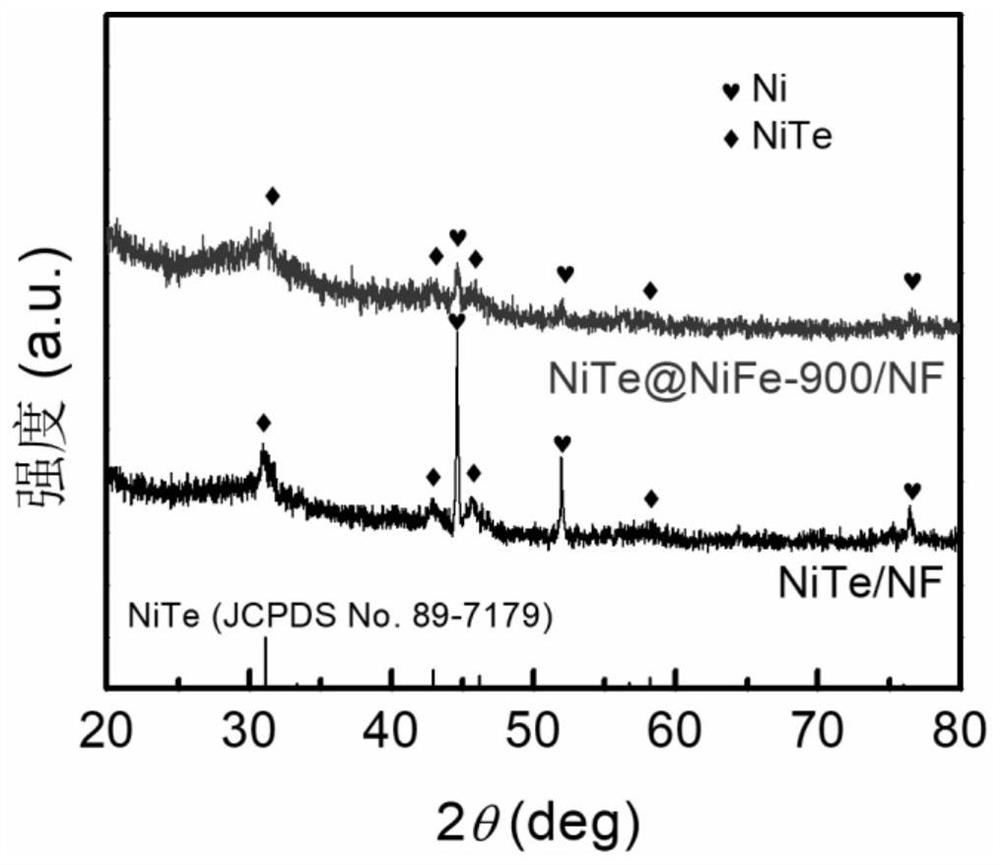
Composite catalyst with NiTe and NiFe loaded on foamed nickel as well as preparation method and application of composite catalyst
A composite catalyst, nickel foam technology, applied in electrodes, electrolysis process, electrolysis components, etc., can solve problems such as poor intrinsic conductivity, limited OER performance, active site exposure, etc., to improve catalytic activity and improve intrinsic conductivity. The effect of poor oxygen evolution rate and optimization of oxygen evolution performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The nickel foam was ultrasonically cleaned with hydrochloric acid and acetone, respectively, and dried in vacuum; 2 TeO 3 Soluble in water, making water and Na 2 TeO 3 The molar ratio is 400:1, stir evenly at room temperature, then add N 2 h 4 ·H 2 O and control N 2 h 4 ·H 2 O and Na 2 TeO 3 The molar ratio is 0.5:1, and after stirring for 5 min, it is transferred to a polytetrafluoroethylene reactor together with foamed nickel, wherein the molar ratio of foamed nickel to the compound containing tellurium is 10:1, followed by hydrothermal reaction After 24 hours, it was naturally cooled and washed, and kept in a vacuum oven at 60°C for 12 hours to obtain NiTe-loaded nickel foam (NiTe / NF).
[0033] 3 mmol FeSO 4 ·7H 2 O and 3 mmol Ni(NO 3 ) 2 ·6H 2 O was dissolved in 200 mL water, NiTe / NF was used as the working electrode, and the deposition time was 900 s. The obtained product was washed and dried in vacuum to obtain the NiTe@NiFe-900 / NF composite catalys...
Embodiment 2
[0039] The nickel foam was ultrasonically cleaned with hydrochloric acid and acetone, dried in vacuum, and Na 2 TeO 3 Soluble in water, making water and Na 2 TeO 3 The molar ratio is 500:1, stir evenly at room temperature, then add N 2 h 4 ·H 2 O and control N 2 h 4 ·H 2 O and Na 2 TeO 3 The molar ratio is 1:1, and after stirring for 5 min, it is transferred to a polytetrafluoroethylene reactor together with foamed nickel, wherein the molar ratio of foamed nickel to the compound containing tellurium is 20:1, followed by hydrothermal reaction After natural cooling for 36 h, it was washed several times with deionized water and absolute ethanol, and kept in a vacuum oven at 60 °C for 12 h to obtain NiTe-loaded nickel foam (NiTe / NF).
[0040] 3mmol Fe(NO 3 ) 3 9H 2 O and 3 mmol Ni(NO 3 ) 2 ·6H 2 O was dissolved in 200 mL of water, NiTe / NF was used as the working electrode, and the deposition time was 700 s. The obtained product was washed and dried in vacuum to obt...
Embodiment 3
[0042] The nickel foam was ultrasonically cleaned with hydrochloric acid and acetone, dried in vacuum, and Na 2 TeO 3 Soluble in water, making water and Na 2 TeO 3 The molar ratio is 600:1, stir well at room temperature, then add N 2 h 4 ·H 2 O and control N 2 h 4 ·H 2 O and Na 2 TeO 3 The molar ratio is 1.5:1. After stirring for 5 min, it is transferred to a polytetrafluoroethylene reactor together with nickel foam. After natural cooling for 12 h, it was washed several times with deionized water and absolute ethanol, and kept in a vacuum oven at 60 °C for 12 h to obtain NiTe-loaded nickel foam (NiTe / NF).
[0043] 3 mmol FeSO 4 ·7H 2 O and 3 mmol Ni(NO 3 ) 2 ·6H 2 O was dissolved in 200 mL of water, NiTe / NF was used as the working electrode, and the deposition time was 1100 s. The obtained product was washed and dried in vacuum to obtain the NiTe@NiFe-1100 / NF composite catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com