a kind of nife 3 n/nf electrochemical catalyst and its preparation method and application
A catalyst and electrochemical technology, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problem of poor electronic conductivity of Ni-based catalysts, incapable of large-scale production, and scarce crustal content, etc. problems, to achieve the effect of low raw material cost, easy operation, and improved oxygen evolution performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] (1) Acid pretreatment: prepare a 3M hydrochloric acid solution, pour it into a single-necked round-bottomed flask, and put it in an oil bath; cut the foamed nickel into a size of 1×2cm, wash it with deionized water, and put it in In a hydrochloric acid flask, heat to 90°C for 30 minutes, then cool to room temperature and rinse with water;
[0046] (2) Surface oxidation treatment: The foamed nickel pretreated with hydrochloric acid is oxidized in the air for 2 days to keep it in a humid state, the temperature is 30°C, and the air humidity is 85%.
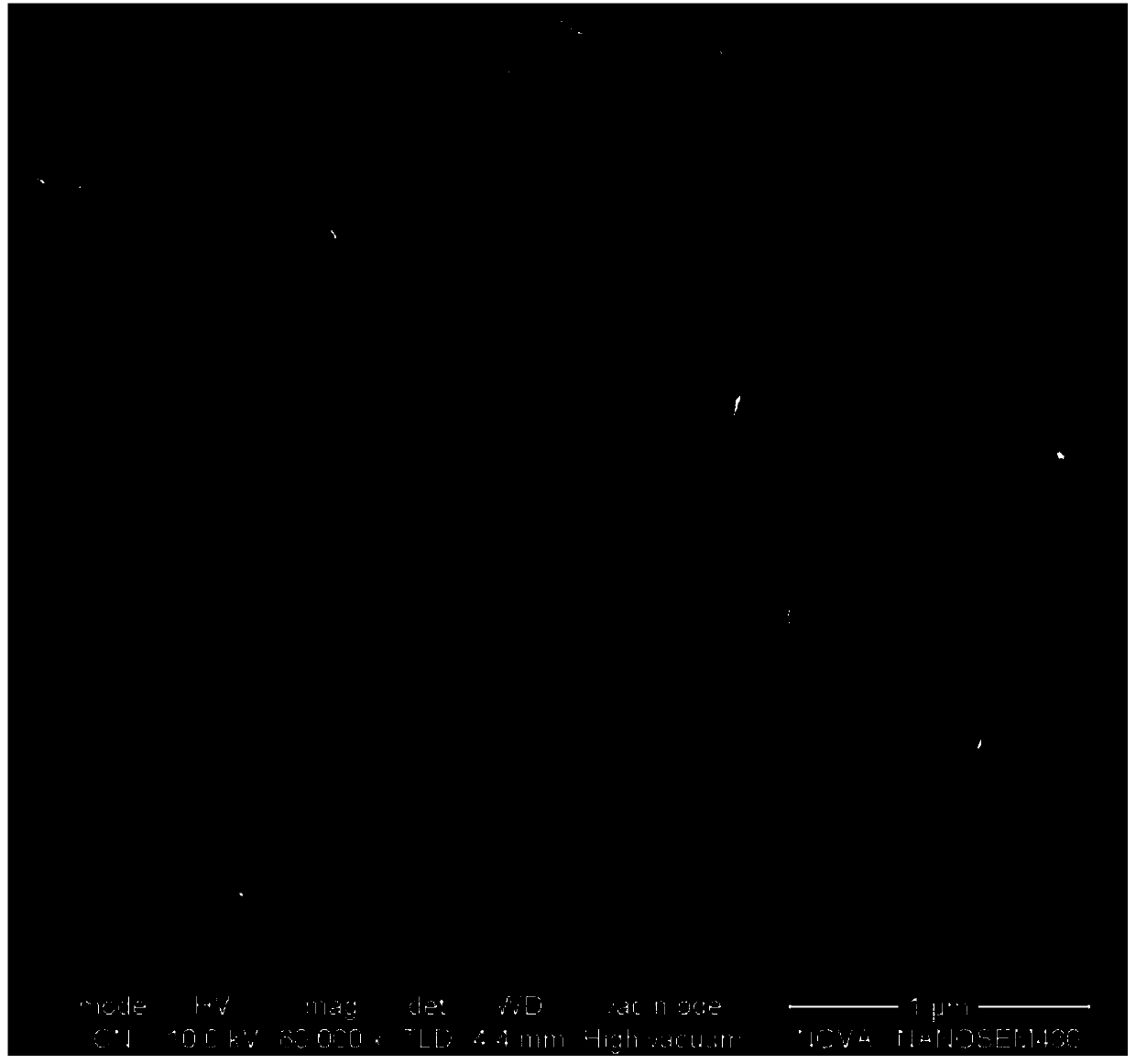
[0047] The morphology of nickel oxide formed on the nickel foam after surface oxidation treatment is as follows figure 1 Shown by figure 1 It can be seen that a thin and uniformly grown nickel oxide layer is formed on the surface of the foamed nickel sheet.
Embodiment 2
[0049] (1) Prepare a 3M hydrochloric acid solution, pour it into a single-neck round-bottomed flask, put it in an oil bath, cut the foamed nickel into a size of 1×2cm, clean it with deionized water, and put it in a flask containing hydrochloric acid In the medium, heated to 90°C for 30 minutes, then cooled to room temperature, and washed with water; the foamed nickel was oxidized in the air for 2 days to keep it in a humid state, the temperature was 30°C, and the air humidity was 85%;
[0050] (2) Prepare 20mL of K with a concentration of 0.02mol / L 4 Fe(CN) 6 Solution, immerse a piece of 1×2cm surface oxidized nickel foam in K 4 Fe(CN) 6 In the solution, add 0.015mol / L trisodium citrate, and use the nickel oxide on the surface of the nickel foam as the nickel source to make the ligand K 4 Fe(CN) 6 Combine the Fe source and the nickel source in the medium, and stand for 6h, 12h, 18h respectively at room temperature to obtain the MOF material (NiFe-PBA / NF);
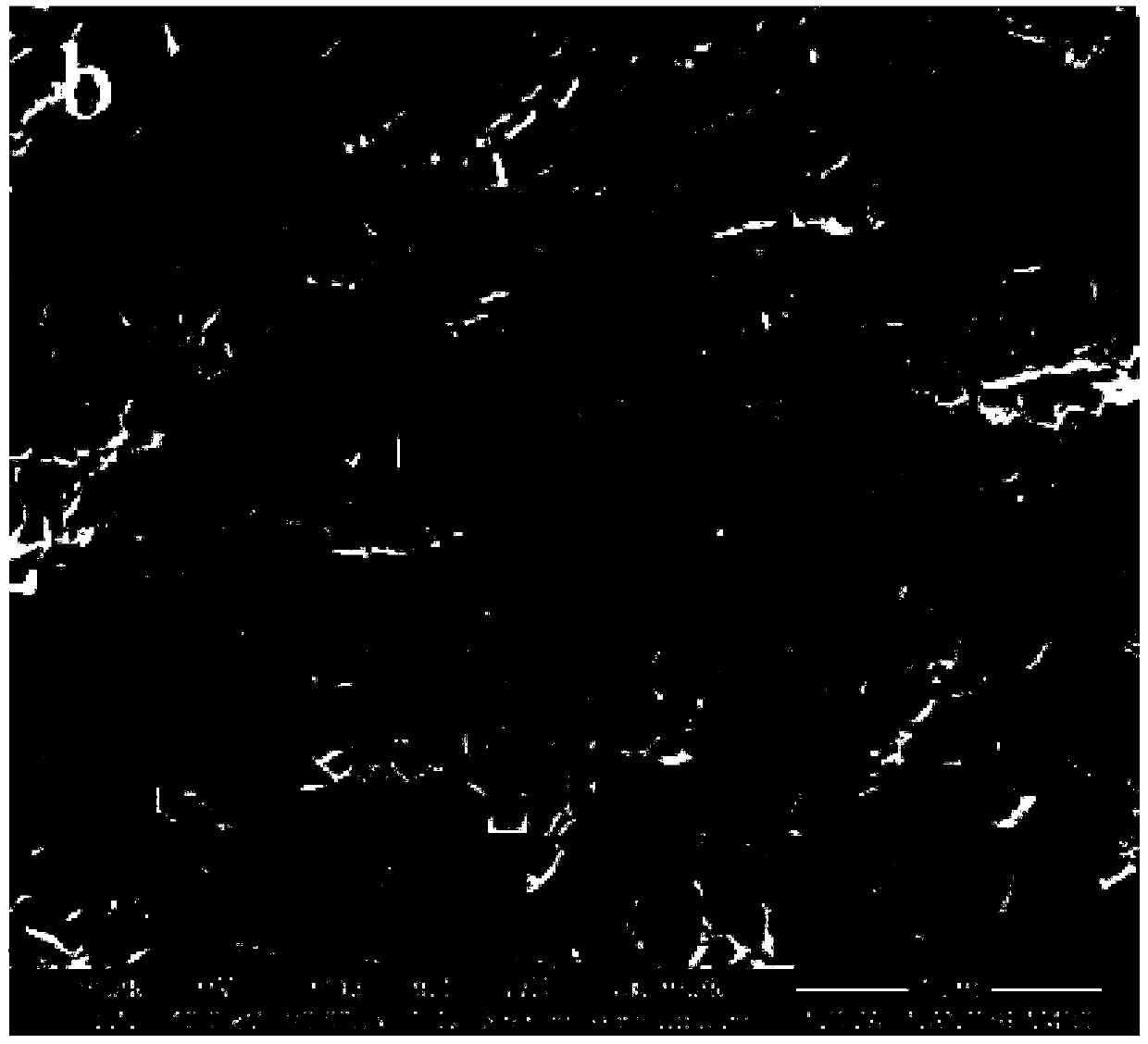
[0051] The SEM image of t...
Embodiment 3
[0053] (1) Prepare a 3M hydrochloric acid solution, pour it into a single-neck round-bottomed flask, put it in an oil bath, cut the foamed nickel into a size of 1×2cm, clean it with deionized water, and put it in a flask containing hydrochloric acid In the medium, heated to 90°C for 30 minutes, then cooled to room temperature, and washed with water; the foamed nickel was oxidized in the air for 2 days to keep it in a humid state, the temperature was 30°C, and the air humidity was 85%;
[0054] (2) Prepare 20mL K with concentrations of 0.01mol / L, 0.015mol / L, 0.02mol / L 4 Fe(CN) 6 Solution, immerse a piece of 1×2cm surface oxidized nickel foam in K 4 Fe(CN) 6 In the solution, add 0.015mol / L trisodium citrate, and use the nickel oxide on the surface of the nickel foam as the nickel source to make the ligand K 4 Fe(CN) 6 Combine the Fe source and the nickel source in, and let stand at room temperature for 12 hours to obtain the MOF material (NiFe-PBA / NF);
[0055] The SEM image of the Ni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com