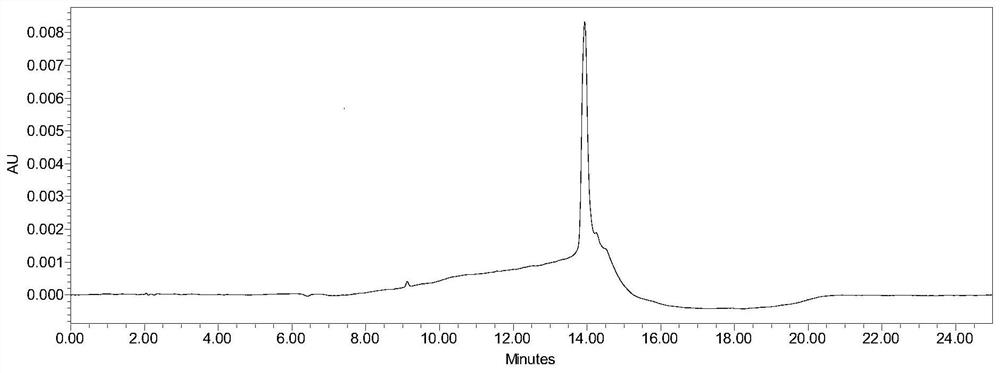
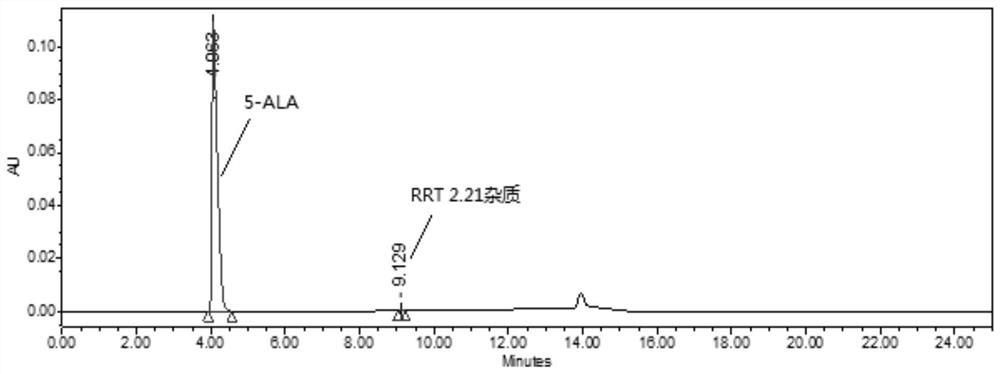
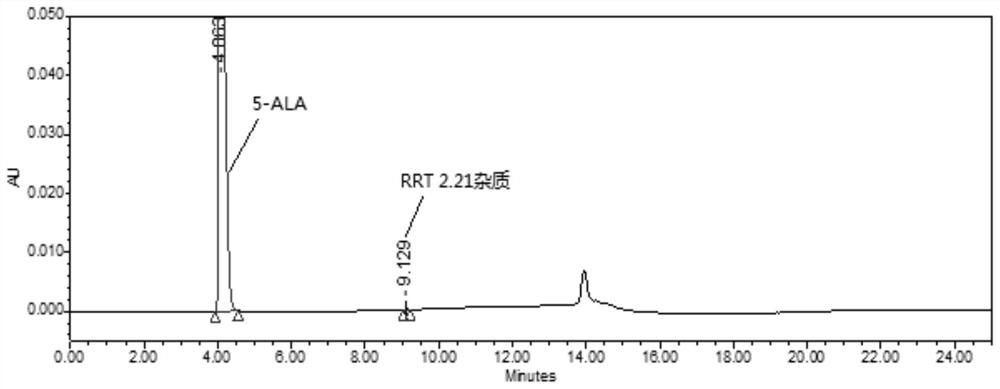
Method for detecting purity of 5-aminolevulinic acid hydrochloride (5-ALA)
A technology of aminolevulinic acid hydrochloride and 5-ALA, applied in the field of chromatographic analysis, can solve problems such as the inability to guarantee the specificity of the detection method and the difficulty of analysis, achieve good technical value and market space, avoid chromatographic interference, and specificity good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0034] A method for detecting the purity of 5-aminolevulinic acid hydrochloride (5-ALA), the method information and related steps include:
[0035] With 0.2% heptafluorobutyric acid aqueous solution as mobile phase A (MPA), with 0.2% heptafluorobutyric acid acetonitrile solution as mobile phase B (MPB), wherein the way of obtaining mobile phase A (MPA) is: 2ml heptafluorobutyrate Add the acid into a solvent bottle containing 1000ml of water, mix well, and perform ultrasonic degassing before use; the way to obtain mobile phase B (MPB) is: add 2ml of heptafluorobutyric acid into a solvent bottle containing 1000ml of acetonitrile, mix well , carry out ultrasonic degassing before use; The heptafluorobutyric acid used in the preparation of mobile phase A (MPA) and mobile phase B (MPB) is selected from liquid chromatography grade, Adamas or equivalent, and acetonitrile is selected from liquid chromatography grade, Scharlau or equivalent, Water is ultrapure water or equivalent.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com