Chiral supramolecular hydrogel element with optimized structure, preparation method and application thereof
A supramolecular hydrogel, chiral amino acid technology, applied in biochemical equipment and methods, general culture methods, microorganisms, etc., can solve the problems of difficult stabilization and enhancement of chiral supramolecular hydrogels, and achieve easy structural Disintegration problems, increased intermolecular forces, effects of simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1, B-pH (D-PHE-OH) shown in Formula (I) 2 Preparation
[0048]
[0049] (1) Benophenylel chloride (3.58 g, 13.0 mmol) was dissolved in dry dichloromethane, and added to two-containing methyl alanine methyl esters (6.0 g, 26.1 mmol). Chloromethane and triethylamine (ET 3 In a solution of N, 8.0 mL, 58.3 mmol, stirred at room temperature, stirred and dissolved in ethanol after stirring the solvent, filtered to obtain B-pH (D-Phe-Ome) 2 .
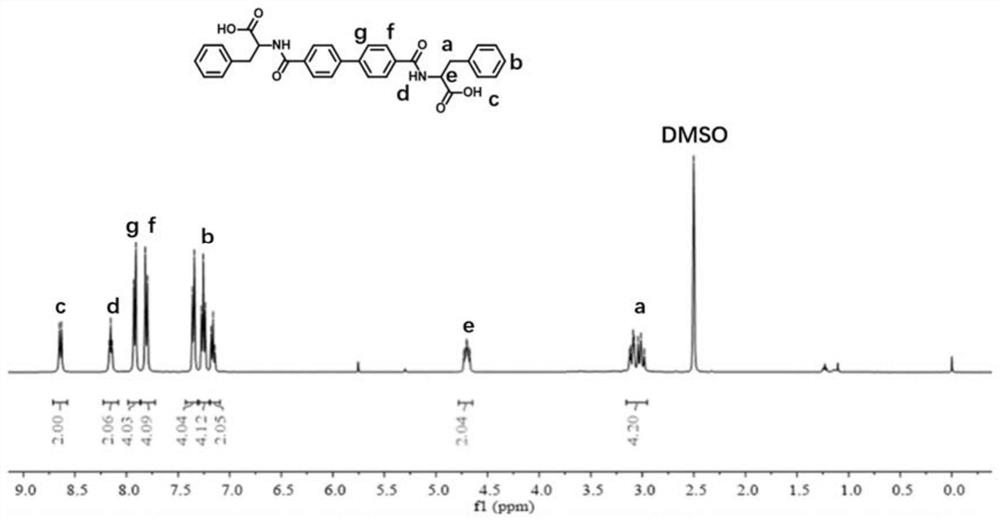
[0050] (2) A NaOH aqueous solution was added to 20 ml of B-pH (D-Phe-OME) 2 (3.46 g, 6.14 mmol) methanol suspension solution, and the aqueous cooled to room temperature was stirred for 24 hours to obtain a clear solution. The gel-shaped precipitate was acidified with EtOAc. The filtration is dried to obtain B-pH (D-Phe-OH) 2 , Nuclear magnetic hydrogen spectrum figure 1 Indicated.
[0051] (3) Preparation of hydrogel: Weigh a certain amount of B-pH (D-PHE-OH) 2 The molecule, add deionized water, after heating to completely dissolv...
Embodiment 2
[0052] Example 2, N-pH (D-PHE-OH) shown in formula (II) 2 Preparation:
[0053]
[0054] (1) 2,6-naphthal chloride (3.27 g, 13.0 mmol) was dissolved in dichloromethane and added to two of D-phenylalanine methyl esters (6.0 g, 26.1 mmol). In a solution of chloromethane and triethylamine (Et3N, 8.0 mL, 58.3 mmol), stirring at room temperature for 24 h, and dissolved in ethanol dissolved after the solvent was evaporated, dried to give B-pH (D-Phe-OME) 2.
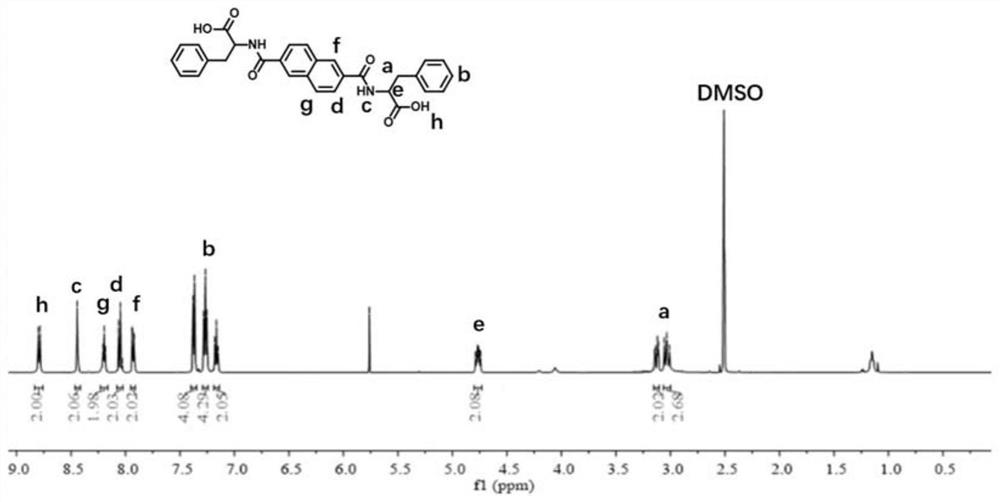
[0055] (2) in 20 ml N-pH (D-Phe-OME) 2 (3.30 g, 6.14 mmol) methanol suspension solution, NaOH aqueous solution was added, and the suction solution was subjected to a clear solution after stirring for 24 hours. The gel-shaped precipitate was acidified with EtOAc. Etch dry and dried to get N-pH (D-PHE-OH) 2 , Nuclear magnetic hydrogen spectrum image 3 Indicated.
[0056] (3) Preparation of hydrogel: Weigh a certain amount of N-pH (D-PHE-OH) 2 The molecule, add deionized water, after heating to completely dissolve, deposit cooling, ...
Embodiment 3
[0057] Example 3, Preparation of PDI-PhoH shown in Formula (III:
[0058]
[0059] (1) Dissolve the osmide dimethyl chloride in dichloromethane, adding to a solution containing D-phenylalanine methyl methyl methyl chloride and triethylamine, stirring at room temperature for 24 h , In the ethanol dissolved after the solvent is removed, the dried dried to obtain a PDI- (PHOME) 2 .
[0060] (2) in PDI- (Phome) 2 In the methanol suspension solution, a NaOH aqueous solution was added, and the suction solution was subjected to a clear solution after slow cooling to room temperature for 24 hours. The gel-shaped precipitate was acidified with EtOAc. The filtration was dried to give PDI-PhoH, and the nuclear magnetic hydrogen spectrum is like Figure 5 Indicated.
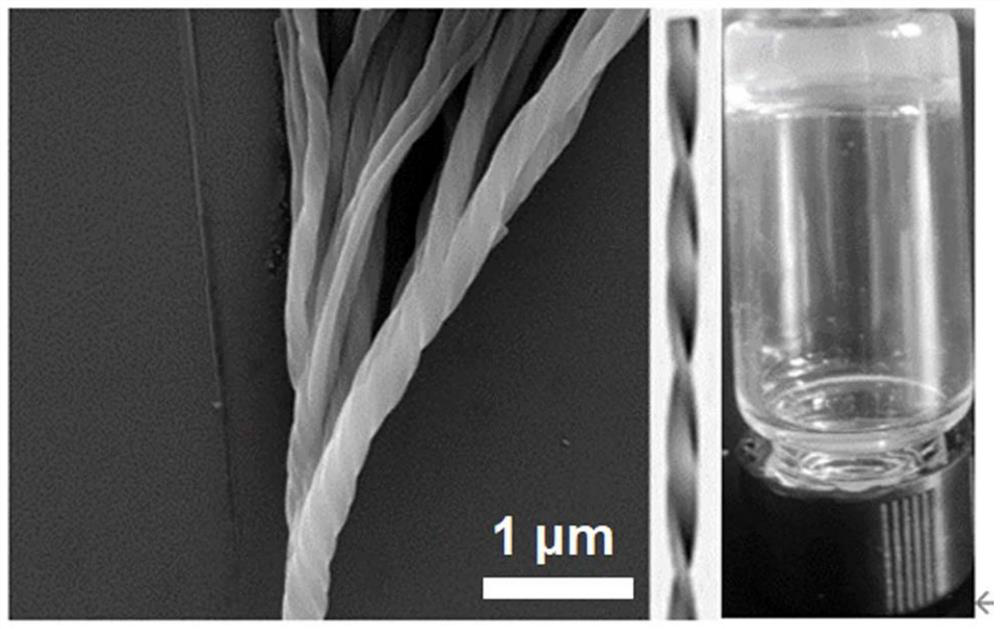
[0061] (3) Preparation of hydrogel: Weigh a certain amount of PDI-PHOH molecules, add deionized water, after heating to completely dissolve, deposition at room temperature, and translucent hydrogel formation. After scanning ele...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com